Write All The Acyclic And Cyclic Isomers Of A Compound Having Molecular
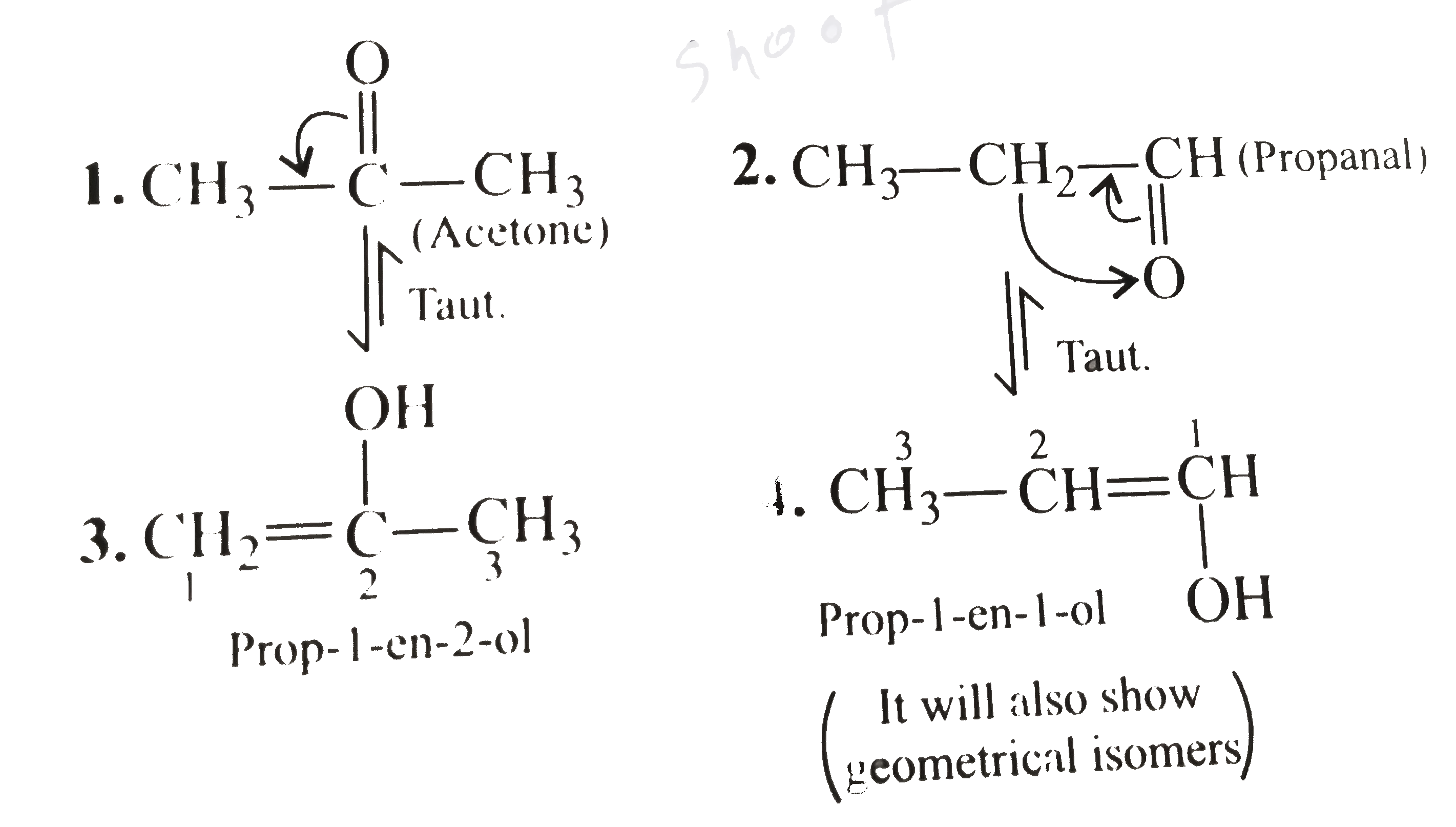
Write All The Acyclic And Cyclic Isomers Of A Compound Having Molecula Draw the structural formula of all the possible isomers of the compound with the molecular formula for c 3 h 6 o and also give their electron dot structures. view solution. q 5. Write down the acyclic isomers having the molecular formula `c 3h 6o`. strategy: first calculate the degree of unsaturation or dbes: `dbe=(sumn(v 2)) asked jun 7, 2019 in chemistry by dikshapatel ( 62.9k points).

Write All The Acyclic And Cyclic Isomers Of A Compound Having Molecula Write all the acyclic and cyclic isomers of a compound having molecular formula c (3)h (6)o.class: 11subject: chemistrychapter: isomerismboard:iit jeeyou can. Step by step video & image solution for write all the acyclic and cyclic isomers of a compound having molecular formula c (3)h (6)o. by chemistry experts to help you in doubts & scoring excellent marks in class 11 exams. Click here👆to get an answer to your question ️ write all the acyclic and cyclic isomers of a compound having molecular formula c3h6o . Write all the acyclic and cyclic isomers of a compound having molecular formula `c (3)h (6)o`. asked may 18, 2019 in chemistry by dikshapatel ( 62.9k points) class 11.

Write All The Acyclic And Cyclic Isomers Of A Compound Having Molecular Click here👆to get an answer to your question ️ write all the acyclic and cyclic isomers of a compound having molecular formula c3h6o . Write all the acyclic and cyclic isomers of a compound having molecular formula `c (3)h (6)o`. asked may 18, 2019 in chemistry by dikshapatel ( 62.9k points) class 11. The total number of acyclic and cyclic isomers including geometrical isomers possible for the molecular formula, c (5) h (10) are. 04:53. view solution. for the method of calculating the degree of unsaturation d.u of c (3)h (6)o = ( (2n (c ) 2) n (h)) (2) = ( (3 xx 2 2) 6) (2) = 1^ (@) a. acyclic isomers [here , (4) will show its position. Draw all the possible cyclic isomers. 1. rings or double bonds. the molecular formula is c3h5br. replacing the br with an equivalent h gives the formula c3h6. the formula of an alkane with 3 carbons is c3h8, so the compound is missing two h atoms. ∴ c3h5br must contain a double bond or a ring. 2.

Comments are closed.