What Is An Fda Pre Submission

Fda Pre Submission All You Need To Know Operon Strategist U.s. food and drug administration center for devices and radiological health document control center (dcc) – wo66 g609 10903 new hampshire avenue silver spring, md 20993 0002. q sub applicants. Information about premarket submissions. premarket notification 510(k) for class i and ii devices that are not exempt from premarket review or are not subject to another type of marketing submission.
Know The Basics About Meeting With The Fda For Medical Device Pre A pre sub is a mechanism for requesting formal written feedback from the fda, and (optionally) a one hour meeting. a pre sub is appropriate when fda’s feedback on specific questions would help guide product development, performance testing, predicate selection, or other aspects of a 510 (k), de novo, pma, or ide submission. Specific premarket submissions, email [email protected]. general small business determination program information, email [email protected]. use cdrh’s customer collaboration. Fda pre submission program is a meeting with the fda where they provide you with feedback before submitting your formal medical device application. it is voluntary, not mandatory, and is a way for you to fix gaps in your application before submitting your final version. a sort of practice exam if you will, with detailed feedback on ways to. Every fda pre submission is a q submission, but not all q submissions are pre submissions. the new prestar template is currently limited to an fda pre submission, but the template will be expanded to other types of q subs later. the fda pre submission template (i.e., prestar) beta version 0.1 is unnecessary for responses to interactive review.

Fda Pre Submission Template Fda pre submission program is a meeting with the fda where they provide you with feedback before submitting your formal medical device application. it is voluntary, not mandatory, and is a way for you to fix gaps in your application before submitting your final version. a sort of practice exam if you will, with detailed feedback on ways to. Every fda pre submission is a q submission, but not all q submissions are pre submissions. the new prestar template is currently limited to an fda pre submission, but the template will be expanded to other types of q subs later. the fda pre submission template (i.e., prestar) beta version 0.1 is unnecessary for responses to interactive review. The q sub program’s origin is in the pre ide feedback system of the mid 1990s. in 2013, the fda launched the pre submission program and expanded pre‑ide communications. as of 2019, the q sub process has widened its reach to include a broad scope of medical device related interactions. Pre submission benefits. the fda cdrh pre submission, or pre sub, is a type of q submission that provides manufacturers an opportunity to communicate with the fda about their planned product submissions. a pre sub allows you to have a meeting with the fda to discuss your plan. some benefits to the pre sub: these meetings may be very beneficial.

A Quick Easy Guide To Fda Pre Submissions The q sub program’s origin is in the pre ide feedback system of the mid 1990s. in 2013, the fda launched the pre submission program and expanded pre‑ide communications. as of 2019, the q sub process has widened its reach to include a broad scope of medical device related interactions. Pre submission benefits. the fda cdrh pre submission, or pre sub, is a type of q submission that provides manufacturers an opportunity to communicate with the fda about their planned product submissions. a pre sub allows you to have a meeting with the fda to discuss your plan. some benefits to the pre sub: these meetings may be very beneficial.
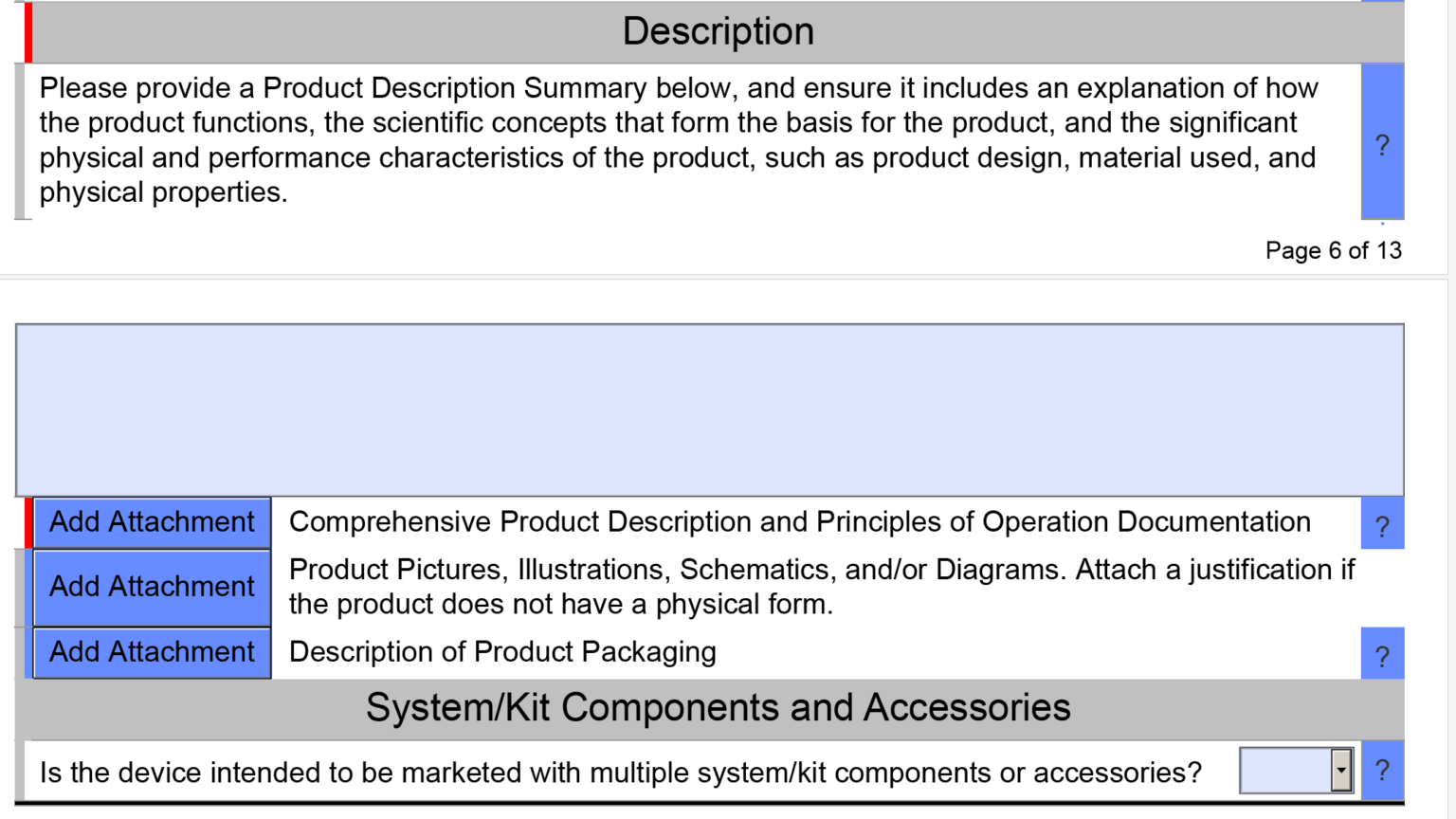
Fda Pre Submission Format And Content Requirements Medical Device Academy

How To Prepare An Fda Pre Submission Free Download

Comments are closed.