Us Fda Medical Device Approval Chart Emergo Group
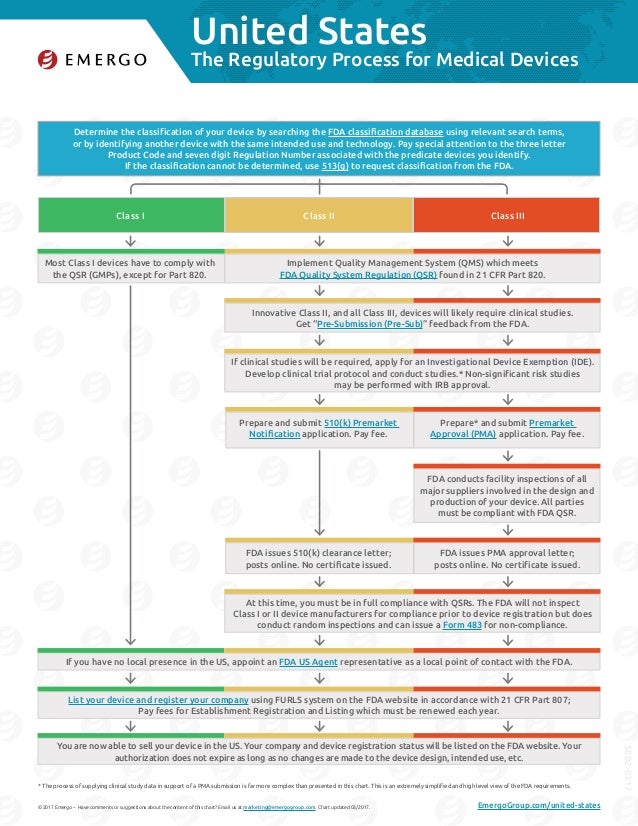
Us Fda Medical Device Approval Chart Emergo Group Upon approval, the fda issues the 510 (k) clearance letter or pma approval letter. step 6. if you have no local presence in the u.s., appoint a u.s. agent as a local point of contact with the fda. step 7. list your device and register your company using the fda unified registration and listing system (furls) on the fda website and pay the. While many devices are easy to classify, others are not. many device classifications require careful evaluation before proceeding with a regulatory submission to the fda. emergo by ul can assist you in classifying your medical device or help you contact the fda for assistance under their 513 (g) request for information (classification) process.

Us Fda Medical Device Approval Chart Emergo This chart illustrates the mda medical device approval process in malaysia and is free to download in the regulatory affairs management suite (rams). page 1 of 2. thanks for your interest in our products and services. let's collect some information so we can connect you with the right person. i would like to receive periodic emails from emergo. Emergo. follow. simple one page chart shows the us fda medical device approval process for class 1, 2 and 3 devices. very easy to understand. read more. 1 of 2. download now. download to read offline. the regulatory process for medical devices in japan involves several key steps: 1) classifying the device and appointing a marketing. A humanitarian use device (hud) is a device that is intended to benefit patients by treating or diagnosing a disease or condition that affects fewer than 8,000 individuals in the united states per. For medical devices in the us, all class iii, class ii, and some class i devices must adhere to design controls outlined in part 820.3 of the quality system regulation (qsr). compliance with 21 cfr part 820 is a prerequisite for fda 510(k) submission, although some low to moderate risk devices may be exempt.

Fda Medical Device Classification Chart A humanitarian use device (hud) is a device that is intended to benefit patients by treating or diagnosing a disease or condition that affects fewer than 8,000 individuals in the united states per. For medical devices in the us, all class iii, class ii, and some class i devices must adhere to design controls outlined in part 820.3 of the quality system regulation (qsr). compliance with 21 cfr part 820 is a prerequisite for fda 510(k) submission, although some low to moderate risk devices may be exempt. Emergo is based in austin, texas and provides quality and regulatory compliance consulting to medical device and ivd companies. the company assists with medical device registration approval, quality management system compliance, in country representation, reimbursement and distributor qualification in 25 markets worldwide. offices in north america, south america, europe, asia, australia and. By stewart eisenhart, emergo group new draft guidance from the us food and drug administration proposes good clinical practice (gcp) compliance for accepting medical device clinical data from.
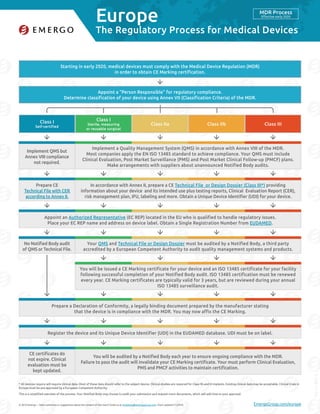
Mdr Compliance Requirements For Medical Devices In Europe Pdf Emergo is based in austin, texas and provides quality and regulatory compliance consulting to medical device and ivd companies. the company assists with medical device registration approval, quality management system compliance, in country representation, reimbursement and distributor qualification in 25 markets worldwide. offices in north america, south america, europe, asia, australia and. By stewart eisenhart, emergo group new draft guidance from the us food and drug administration proposes good clinical practice (gcp) compliance for accepting medical device clinical data from.

Comments are closed.