Updated Results Of The Bruin Trial Pirtobrutinib In R R Mcl
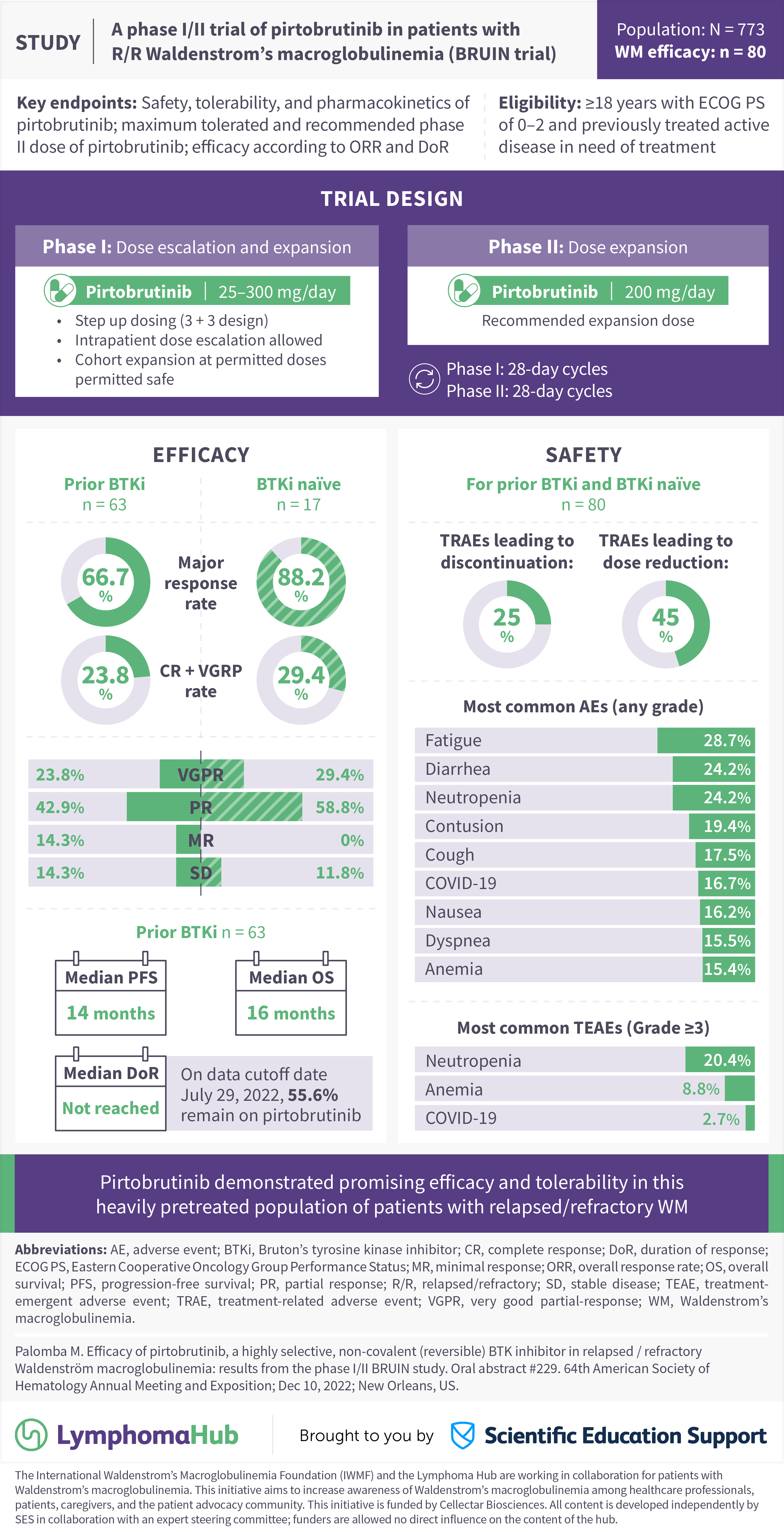
A Phase I Ii Trial Of Pirtobrutinib In Patients With R R Waldenstrom S Here, we report updated results of pirtobrutinib therapy in all patients (pts), including those with biologically high risk r r mcl with a median survival follow up of 24.2 months (range, 18.2 29.8). methods: pts with r r mcl received pirtobrutinib monotherapy in the multicenter phase 1 2 bruin trial (nct03740529). efficacy was assessed in all. Here, we report updated results of pirtobrutinib in patients (pts) with cbtki pre treated relapsed refractory (r r) mcl and more than 3 years follow up from start of enrollment. methods: pts with cbtki pre treated r r mcl received pirtobrutinib monotherapy in a multicenter phase 1 2 bruin trial (nct03740529). efficacy was assessed in the.

Updated Results Of The Bruin Trial Pirtobrutinib In R R Mcl Youtube We report the safety and efficacy of pirtobrutinib in patients with covalent bruton tyrosine kinase inhibitor (cbtki) pretreated mantle cell lymphoma (mcl), a population with poor prognosis. methods: patients with cbtki pretreated relapsed refractory (r r) mcl received pirtobrutinib monotherapy in a multicenter phase i ii trial (bruin. Patients with relapsed or refractory b cell cancers were eligible for treatment with pirtobrutinib in the phase 1–2 bruin trial, the results of which have been previously published in part. 22. Patients with r r mcl and other b cell malignancies, including those who were previously treated with a cbtki, were eligible for treatment with pirtobrutinib monotherapy in the first in human open label, multicenter, phase i ii bruin trial. 19 patient allocation by b cell malignancy is included in the data supplement ([fig s1], online only). The objective of this study is to establish whether pirtobrutinib is superior to investigator’s choice of cbtki in pts with previously treated, btki naïve mcl. methods: bruin mcl 321 (nct04662255) is a randomized, open label, global phase 3 study comparing pirtobrutinib monotherapy versus investigator’s choice of cbtki monotherapy.

Visualabstract Pirtobrutinib Is Safe And Efficacious In The Treatment Patients with r r mcl and other b cell malignancies, including those who were previously treated with a cbtki, were eligible for treatment with pirtobrutinib monotherapy in the first in human open label, multicenter, phase i ii bruin trial. 19 patient allocation by b cell malignancy is included in the data supplement ([fig s1], online only). The objective of this study is to establish whether pirtobrutinib is superior to investigator’s choice of cbtki in pts with previously treated, btki naïve mcl. methods: bruin mcl 321 (nct04662255) is a randomized, open label, global phase 3 study comparing pirtobrutinib monotherapy versus investigator’s choice of cbtki monotherapy. The bruin mcl 321 study compares pirtobrutinib with three currently approved covalent btk inhibitors (ibrutinib, acalabrutinib or zanubrutinib), in patients with mcl who have never received any form of btk inhibitor. this trial will look at how many people live with the disease without it getting worse. Pirtobrutinib was well tolerated and exhibited a wide therapeutic index. updated data, including approximately 60 new patients with mcl and an additional 10 months since the prior data cut will be presented. keywords: mcl, pirtobrutinib, btki, mantle cell lymphoma, clinical trial, phase i ii mcl 135 bruin mcl 321, a phase 3 open.

A Phase I Ii Trial Of Pirtobrutinib In Patients With R R Waldenstrom S The bruin mcl 321 study compares pirtobrutinib with three currently approved covalent btk inhibitors (ibrutinib, acalabrutinib or zanubrutinib), in patients with mcl who have never received any form of btk inhibitor. this trial will look at how many people live with the disease without it getting worse. Pirtobrutinib was well tolerated and exhibited a wide therapeutic index. updated data, including approximately 60 new patients with mcl and an additional 10 months since the prior data cut will be presented. keywords: mcl, pirtobrutinib, btki, mantle cell lymphoma, clinical trial, phase i ii mcl 135 bruin mcl 321, a phase 3 open.

Ash 2022 Efficacy Of Pirtobrutinib A Highly Selective Non Covalent

Comments are closed.