Thermodynamics What Do Heat And Work Really Mean Basics Of Thermodynamics
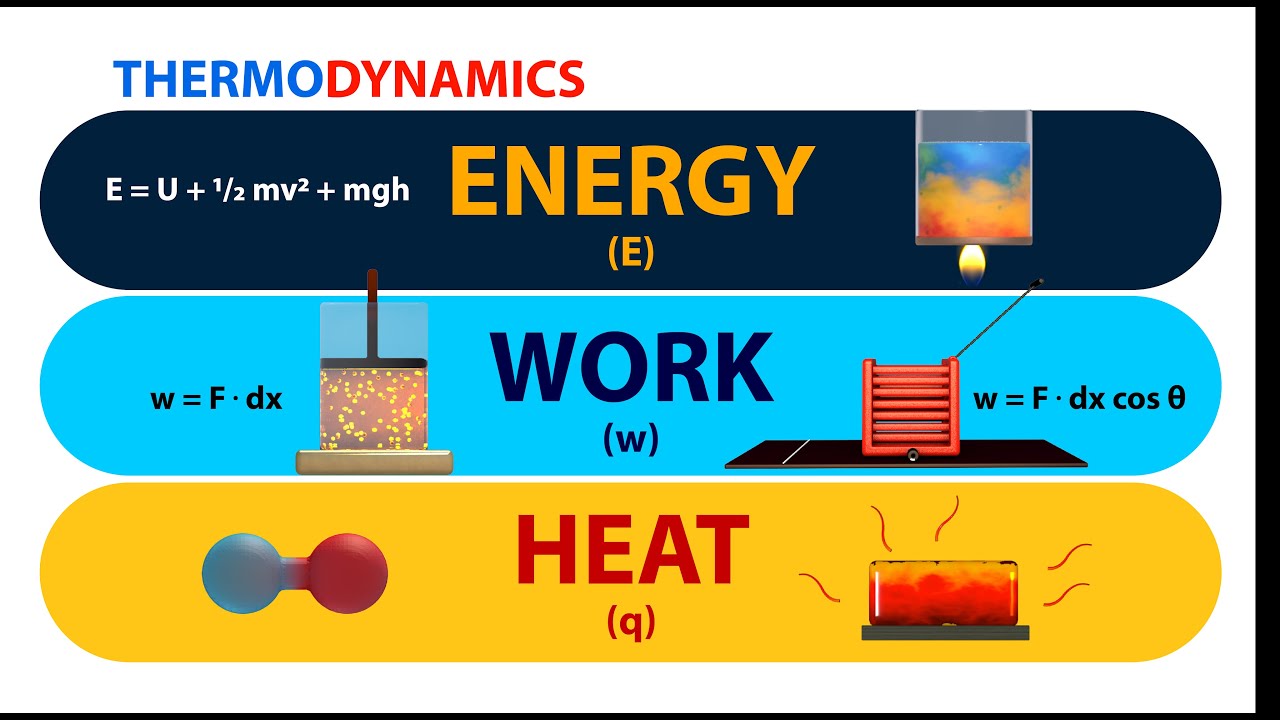
Thermodynamics Energy Work And Heat Animation Youtube "work" and "heat" are commonly used words in everyday life. but they mean very specific things in the physics field of thermodynamics.hey everyone, in this (. Thermodynamics is the science of the relationship between heat, work, temperature, and energy. heat was not formally recognized as a form of energy until about 1798, when count rumford (sir benjamin thompson), a british military engineer, noticed that limitless amounts of heat could be generated in the boring of cannon barrels and that the.

4 Laws Of Thermodynamics With Examples Very Simple Energy is transferred along with the genetic material and so obeys the first law of thermodynamics. energy is transferred—not created or destroyed—in the process. when work is done on a cell or heat transfers energy to a cell, the cell’s internal energy increases. when a cell does work or loses heat, its internal energy decreases. Thermodynamics is the study of the relationship between heat (or energy) and work. in other words, thermodynamics looks at how we can put energy into a system (whether it is a machine or a molecule) and make it do work. alternatively, we might be able to do some work on a system and make it produce energy (like spinning the turbines in a power. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. in equation form, the first law of thermodynamics is. Δu = q − w. (15.1.1) (15.1.1) Δ u = q − w. here Δu Δ u is the change in internal energy u u of the system. The laws of thermodynamics describe the relationship between matter and energy and how they relate to temperature and entropy. many texts list the three laws of thermodynamics, but really there are four laws (although the 4th law is called the zeroeth law). here’s a list of the laws of thermodynamics and a quick summary of what each law means.

Laws Of Thermodynamics The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. in equation form, the first law of thermodynamics is. Δu = q − w. (15.1.1) (15.1.1) Δ u = q − w. here Δu Δ u is the change in internal energy u u of the system. The laws of thermodynamics describe the relationship between matter and energy and how they relate to temperature and entropy. many texts list the three laws of thermodynamics, but really there are four laws (although the 4th law is called the zeroeth law). here’s a list of the laws of thermodynamics and a quick summary of what each law means. Thermodynamics is the field of physics that deals with the relationship between heat and other properties (such as pressure, density, temperature, etc.) in a substance. specifically, thermodynamics focuses largely on how a heat transfer is related to various energy changes within a physical system undergoing a thermodynamic process. A continuous succession of equilibrium states is known as a thermodynamic path, which can be represented as a smooth curve in a multidimensional space whose axes are labeled by state variables. a thermodynamic process is any change or succession of changes which results in a change of the state variables.

First Law Of Thermodynamics Thermal Energy And Work Done Youtube Thermodynamics is the field of physics that deals with the relationship between heat and other properties (such as pressure, density, temperature, etc.) in a substance. specifically, thermodynamics focuses largely on how a heat transfer is related to various energy changes within a physical system undergoing a thermodynamic process. A continuous succession of equilibrium states is known as a thermodynamic path, which can be represented as a smooth curve in a multidimensional space whose axes are labeled by state variables. a thermodynamic process is any change or succession of changes which results in a change of the state variables.

First Law Of Thermodynamics Basic Introduction Internal Energy Heat

Comments are closed.