Thermodynamics Energy Work And Heat Animation Youtube
Thermodynamics Energy Work And Heat Animation Youtube #thermodynamicschemistry #energy #kineticschool thermodynamics: energy, work and heat (animation)chapter:0:00 intro0:17 energy1:10 work2:05 heat2:37 heat a. We've all heard of the laws of thermodynamics, but what are they really? what the heck is entropy and what does it mean for the fate of the universe? how doe.

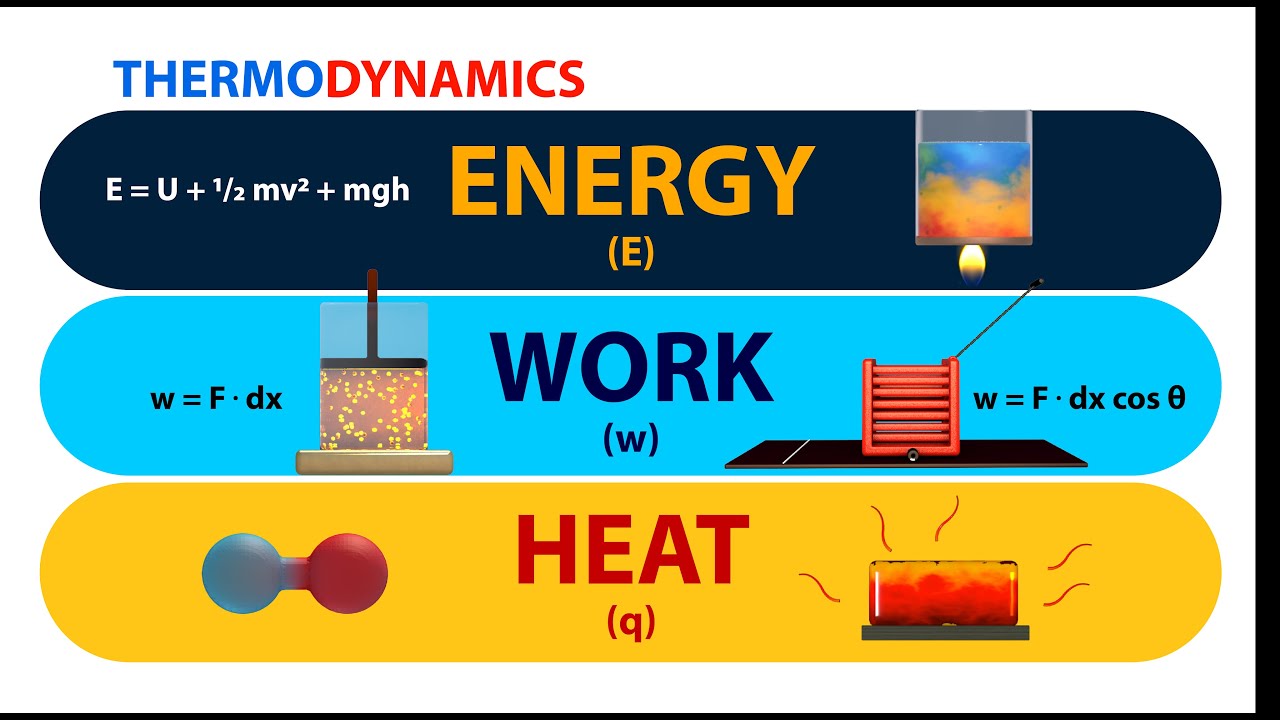
First Law Of Thermodynamics Basic Introduction Internal Energy Heat Learn to differentiate between energy transfer by heat and work in closed systems. we discuss about what a system is, boundaries, heat and work. find more at. The si unit for energy is the "joule." thermodynamics: energy, work, and heat "energy is the quantitative property that must be transferred to a body or physical system to perform work on the body or to heat it." work in thermodynamics. work in thermodynamics involves the transfer of energy between mechanical systems. energy is a quantitative. Unit 7 the energy in chemical reactions: thermodynamics and enthalpy. by first looking at work and heat, the course adds another dimension: the energetics of chemical reactions. this study of thermodynamics can lead to predicting how chemical reactions will proceed or how much energy is required or released during the reactions. Heat is a form of energy, which flows due to difference in temperature. it flows from high temperature to low temperature. heat is a path function, ie, it depends on the path followed by the system to reach to the current state. sign convention: heat absorbed by the system is taken as positive and heat released by the system is taken as negative.

Energy Work And Heat Youtube Unit 7 the energy in chemical reactions: thermodynamics and enthalpy. by first looking at work and heat, the course adds another dimension: the energetics of chemical reactions. this study of thermodynamics can lead to predicting how chemical reactions will proceed or how much energy is required or released during the reactions. Heat is a form of energy, which flows due to difference in temperature. it flows from high temperature to low temperature. heat is a path function, ie, it depends on the path followed by the system to reach to the current state. sign convention: heat absorbed by the system is taken as positive and heat released by the system is taken as negative. In chemistry we talked about the first law of thermodynamics as being the law of conservation of energy, and that's one way of looking at it, but physicists. Thermodynamics deals with processes which involve energy changes as a result of heat flow to or from a system and or work done on or by a system. often the ‘system’ considered is a fixed mass of gas separated from its surroundings by a cylinder and a piston: the energy in a system is called its internal energy.

Comments are closed.