Thermodynamics 14 Comparison Of Heat And Work
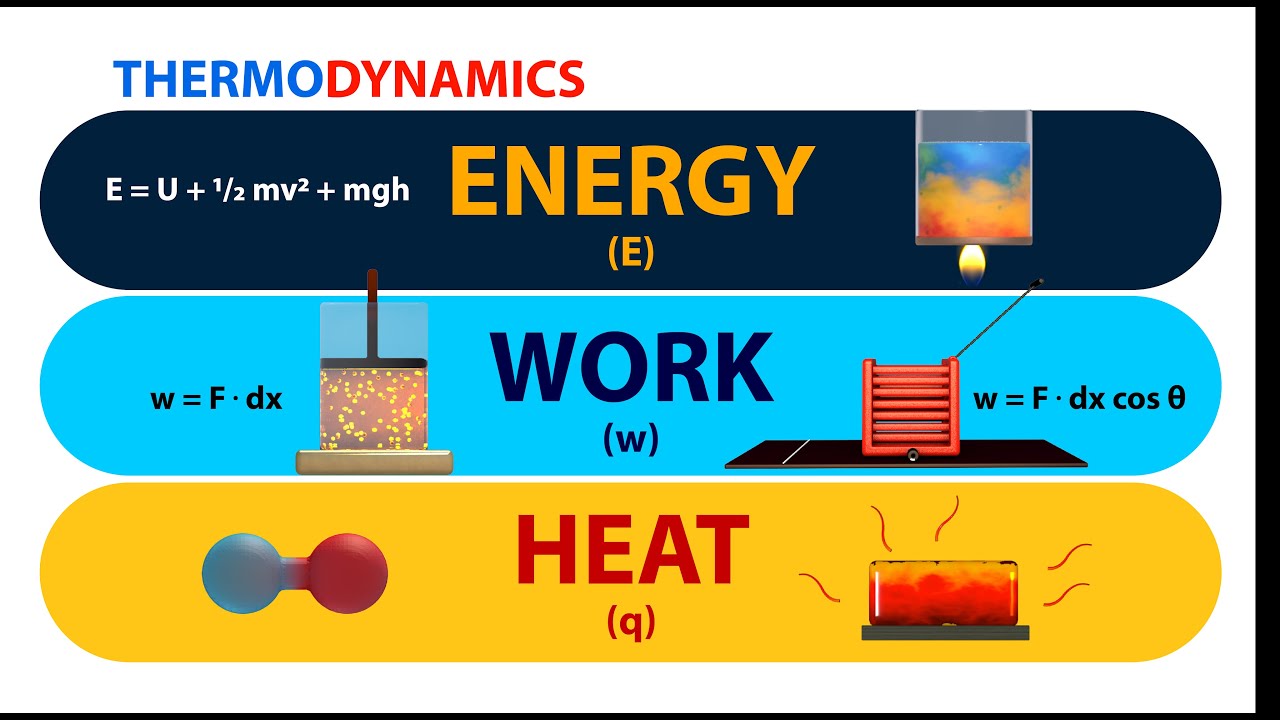
Thermodynamics Energy Work And Heat Animation Youtube The maximum amount of work one can attain from heat is given by the carnot efficiency. heat is the energy associated with the random motion of particles, while work is the energy of ordered motion in one direction. therefore heat is "low quality" energy and work is "high quality" energy, and this supports the entropy statement of the second law. While both involve energy transfer, they differ in their mechanisms, effects, quantification, and conversion. heat is the transfer of energy due to a temperature difference, while work is the transfer of energy due to the application of a force. understanding the attributes of heat and work is essential in comprehending the behavior of physical.

Difference Between Heat And Work Heat And Work Difference Youtube Figure 14.3.1 14.3. 1 shows a gas confined to a cylinder that has a movable piston at one end. if the gas expands against the piston, it exerts a force through a distance and does work on the piston. if the piston compresses the gas as it is moved inward, work is also done—in this case, on the gas. Figure 12.6 the first law of thermodynamics is the conservation of energy principle stated for a system, where heat and work are the methods of transferring energy to and from a system. q represents the net heat transfer—it is the sum of all transfers of energy by heat into and out of the system. Energy is measured in terms of its ability to perform work or to transfer heat. mechanical work is done when a force f displaces an object by a distance d: w = f × d. the basic unit of energy is the joule. one joule is the amount of work done when a force of 1 newton acts over a distance of 1 m; thus 1 j = 1 n m. Thermodynamics is the study of the connection between heat and work and the conversion of one into the other. this study is important because many machines and modern devices change heat into work (such as an automobile engine) or turn work into heat (or cooling, as in a refrigerator). there are two laws of thermodynamics that explain the connection between work and heat.
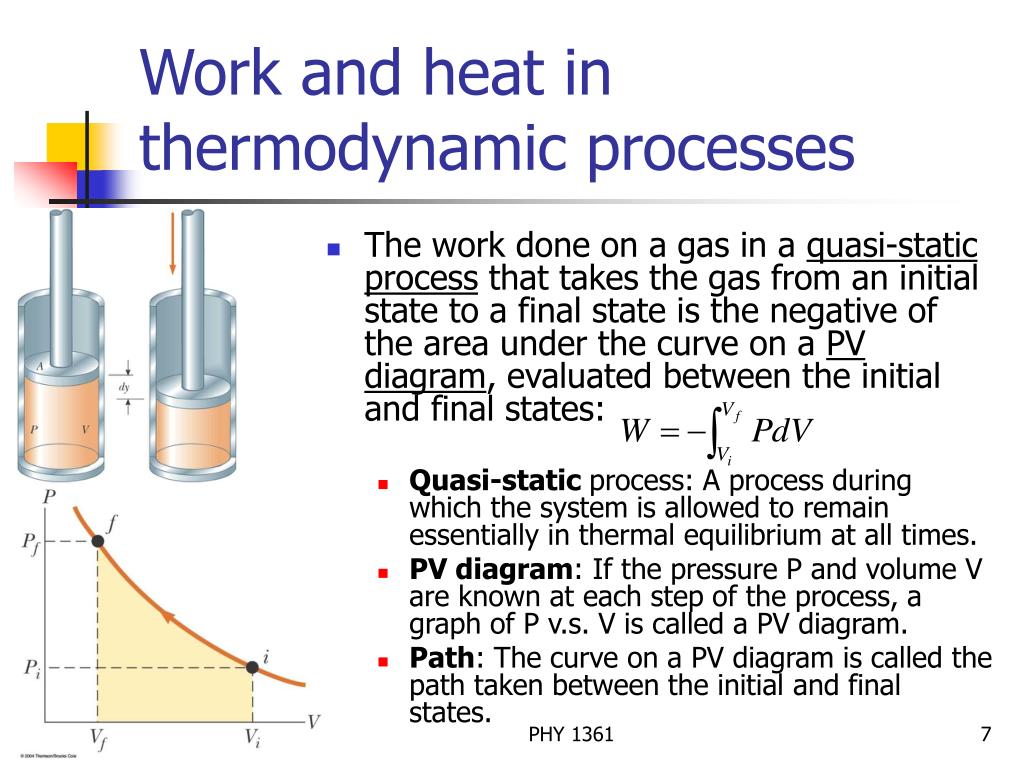
Ppt Chapter 20 Powerpoint Presentation Free Download Id 822227 Energy is measured in terms of its ability to perform work or to transfer heat. mechanical work is done when a force f displaces an object by a distance d: w = f × d. the basic unit of energy is the joule. one joule is the amount of work done when a force of 1 newton acts over a distance of 1 m; thus 1 j = 1 n m. Thermodynamics is the study of the connection between heat and work and the conversion of one into the other. this study is important because many machines and modern devices change heat into work (such as an automobile engine) or turn work into heat (or cooling, as in a refrigerator). there are two laws of thermodynamics that explain the connection between work and heat. Chapter 3. t and the first law of thermodynamics3.1 mechanical workmechanical work is defined as an energy transfer. o the system through the change of an external parameter. work is the only energy which is tran. mal volume change (reversible transfor ma. ion)dv = adx ,where a is the active area of the piston. in equ. The internal energy (u) of a system is a measure of its capacity to supply energy that can do work within the surroundings, making u the ideal variable to keep track of the flow of heat and work energy into and out of a system. changes in the internal energy of a system (Δu) can be calculated by. Δu = uf − ui.
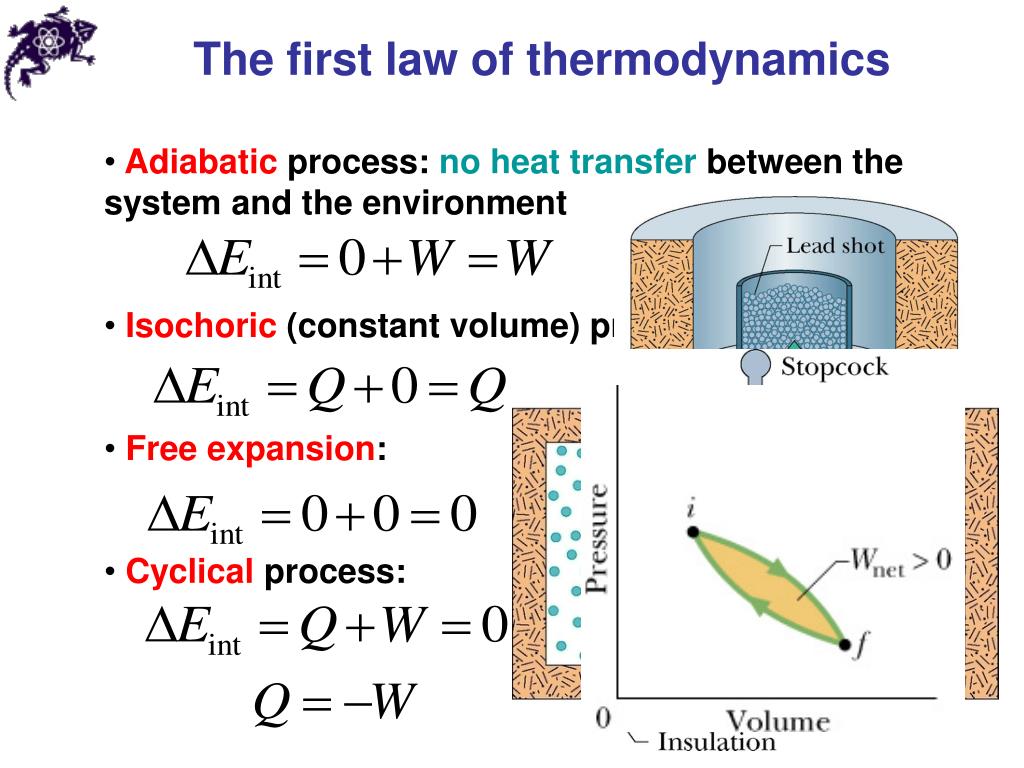
Ppt Chapters 19 20 Temperature Heat And The First Law Of Chapter 3. t and the first law of thermodynamics3.1 mechanical workmechanical work is defined as an energy transfer. o the system through the change of an external parameter. work is the only energy which is tran. mal volume change (reversible transfor ma. ion)dv = adx ,where a is the active area of the piston. in equ. The internal energy (u) of a system is a measure of its capacity to supply energy that can do work within the surroundings, making u the ideal variable to keep track of the flow of heat and work energy into and out of a system. changes in the internal energy of a system (Δu) can be calculated by. Δu = uf − ui.

Comments are closed.