Theoretical Yield In Grams

How To Calculate Theoretical Yield 12 Steps With Pictures Finally, the theoretical yield is the number of moles of oxygen gas multiplied by the moles to grams conversion factor: theoretical yield of o 2 = (9 moles)(32 grams mole) theoretical yield of o 2 = 288 grams. calculate reactant needed to make product. a variation of the theoretical yield calculation helps you find how much reactant you use. 5. convert the result to grams. this is the reverse of your earlier step of calculating the number of moles or reactant. when you know the number of moles that you expect, you will multiply by the molar mass of the product to find the theoretical yield in grams.
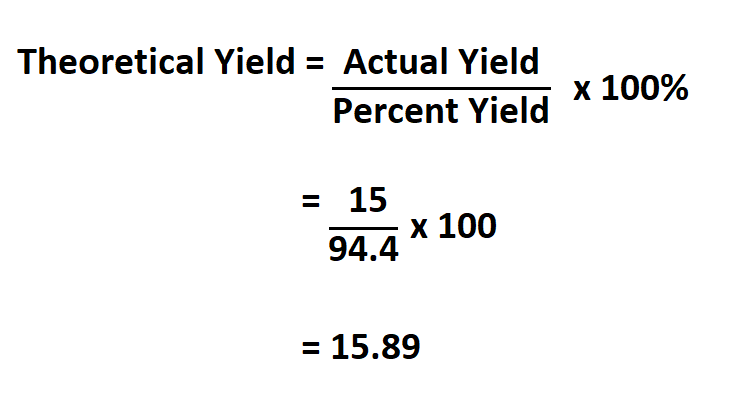
How To Calculate Theoretical Yield The theoretical yield of co 2 depends on the reaction taking place and the amount of reagents. to find the theoretical yield, you can follow the steps below: find the moles of the limiting reagent. multiply the moles of the limiting reagent by the stoichiometry of carbon dioxide in the reaction to give the moles of co 2 produced. Theoretical yield formula quick review . balance your equations. find the mole ratio between the reactant and the product. calculate using the following strategy: convert grams to moles, use the mole ratio to bridge products and reactants, and then convert moles back to grams. Convert the amount of each reactant and product you are working with into moles, if you are provided the amount in grams. to find the number of moles, divide the amount in grams by the molar mass you calculated in step 2. identify the limiting reactant. look at the ratios of reactant to product you obtained in step 3, and then look at how much. Percent yield = actual yield theoretical yield × 100%. use the percent yield equation above. step 2: solve. percent yield = 14.9 g 15.7 g × 100% = 94.9%. step 3: think about your result. since the actual yield is slightly less than the theoretical yield, the percent yield is just under 100%.
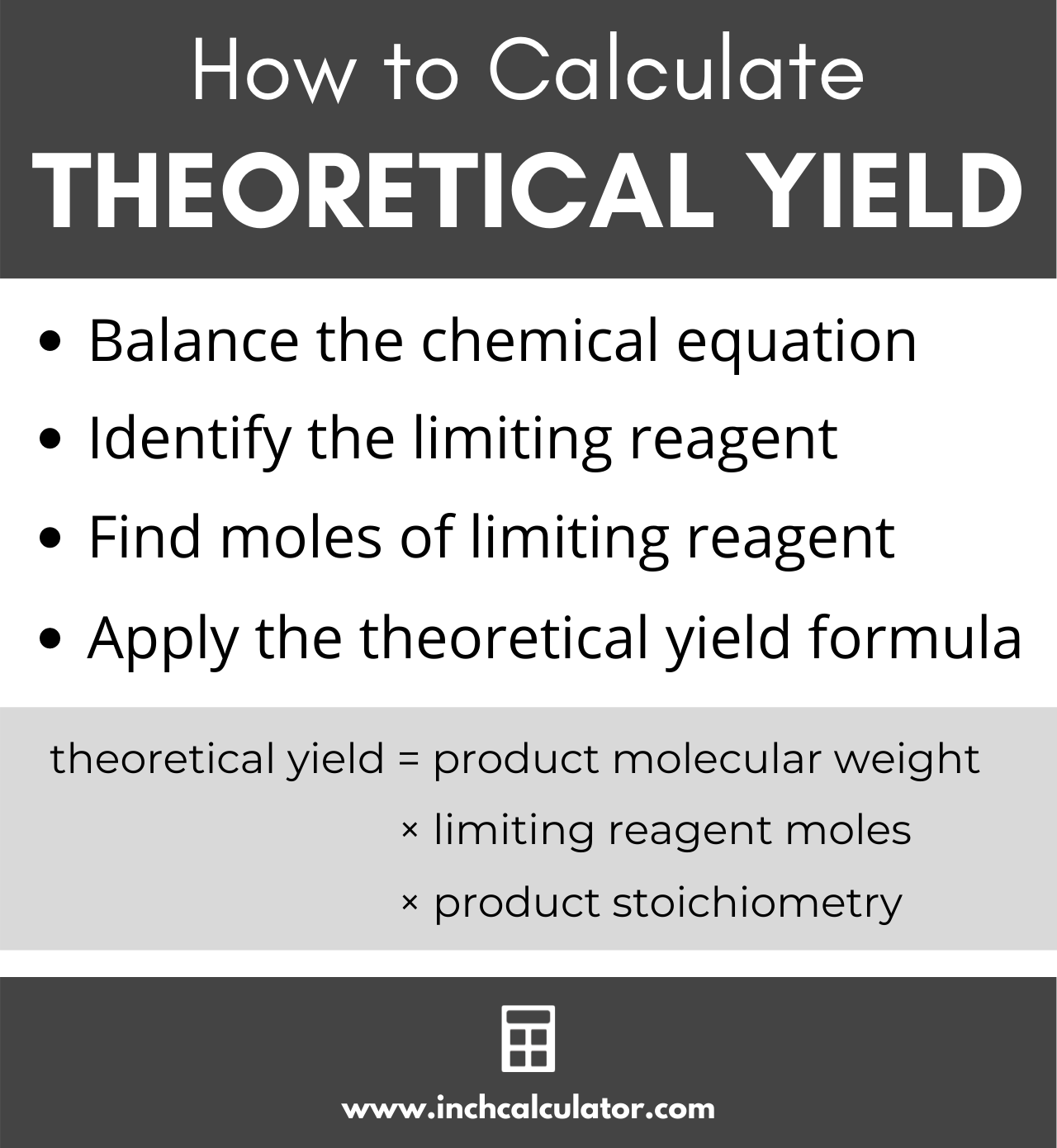
Theoretical Yield In Grams Convert the amount of each reactant and product you are working with into moles, if you are provided the amount in grams. to find the number of moles, divide the amount in grams by the molar mass you calculated in step 2. identify the limiting reactant. look at the ratios of reactant to product you obtained in step 3, and then look at how much. Percent yield = actual yield theoretical yield × 100%. use the percent yield equation above. step 2: solve. percent yield = 14.9 g 15.7 g × 100% = 94.9%. step 3: think about your result. since the actual yield is slightly less than the theoretical yield, the percent yield is just under 100%. Our theoretical yield calculator employs the fundamental principles of stoichiometry and chemistry. here are the key formulas it uses: ty = \dfrac {\text {limiting reagent mass (g)} \cdot \text {molar mass of product (g mol)}} {\text {molecular weight of limiting reagent (g mol)} \cdot \text {stoichiometry}} t y = molecular weight of limiting. The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100 to give a percentage: percent yield = actual yield (g) theoretical yield(g) × 100%. the method used to calculate the percent yield of a reaction is illustrated in example 4.3.4.

Theoretical Yield In Grams Our theoretical yield calculator employs the fundamental principles of stoichiometry and chemistry. here are the key formulas it uses: ty = \dfrac {\text {limiting reagent mass (g)} \cdot \text {molar mass of product (g mol)}} {\text {molecular weight of limiting reagent (g mol)} \cdot \text {stoichiometry}} t y = molecular weight of limiting. The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100 to give a percentage: percent yield = actual yield (g) theoretical yield(g) × 100%. the method used to calculate the percent yield of a reaction is illustrated in example 4.3.4.

Comments are closed.