Stool Test For Colon Cancer Screening Ohio State Health Discovery

Stool Test For Colon Cancer Screening Ohio State Health Discovery Schedule a screening colonoscopy at ohio state. call 614 293 6255 to schedule your colonoscopy today. subhankar chakraborty, md, phd, is a gastroenterologist at the ohio state university wexner medical center and a clinical assistant professor of internal medicine at the ohio state university college of medicine. When should you be screened for colorectal cancer? the current guideline is to begin screening at age 45 for average risk people. average risk is defined as having no family history of colon cancer or high risk polyps. high risk individuals are defined as people with a first degree relative (parent, sibling or child) diagnosed with colon cancer.

Colorectal Cancer About 30% of those diagnosed with colorectal cancer at a younger age have a hereditary predisposition for cancer. this can include having close relatives diagnosed with colorectal cancer or other forms of cancer, such as uterine, ovarian or stomach cancer. rare syndromes, like lynch syndrome, increase a person’s risk of getting cancer. Use the tip of the wand (attached to the cap of the collection bottle) to scrape the surface of the stool before it touches the water. cover the grooved portion of the wand completely with stool. What the results mean: if a stool dna test finds something abnormal, a colonoscopy may be needed to follow up on the findings. your doctor should have the results within two weeks from the time that the sample is received at the lab. cost: stool dna tests are more expensive than other stool based tests. if it’s not covered by insurance, the. The american cancer recommends that adults should begin colorectal cancer screening at age 50 using one of the following tests: flexible sigmoidoscopy (every five years), colonoscopy (every 10.
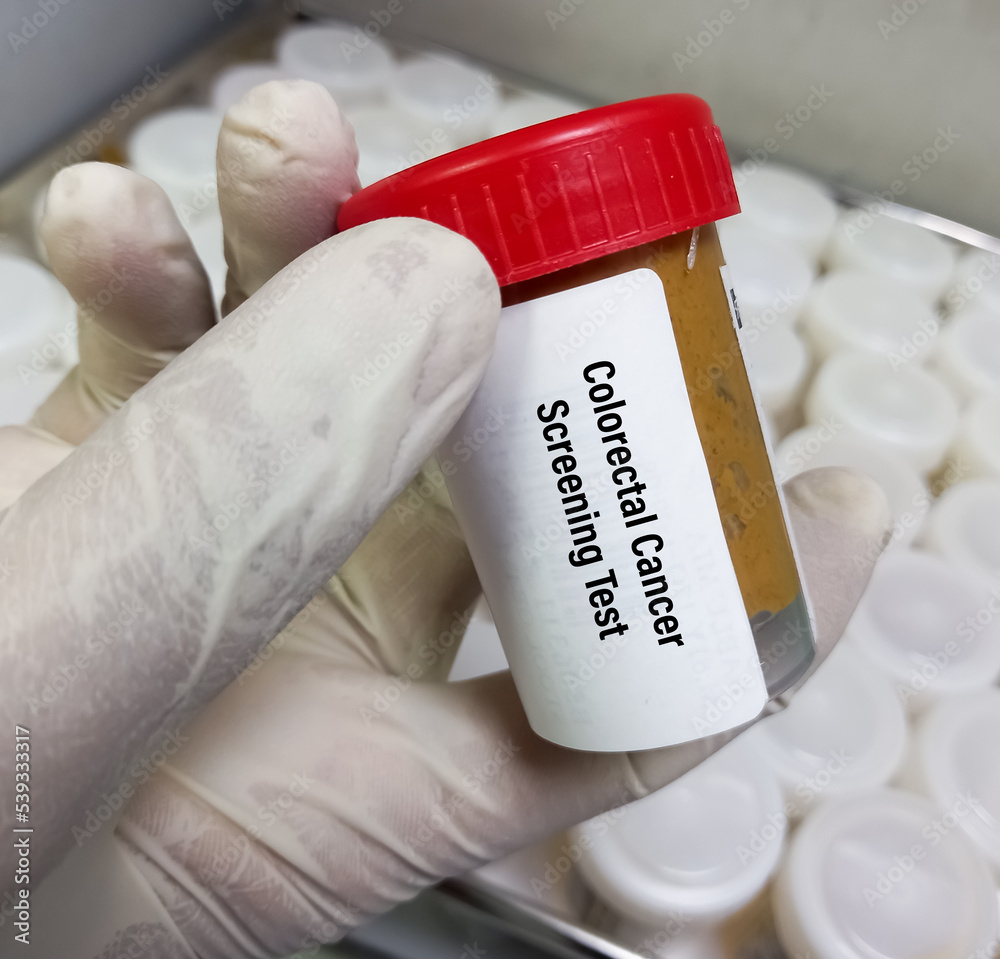
Stool Sample For Colorectal Cancer Screening Test Stool Based Tests What the results mean: if a stool dna test finds something abnormal, a colonoscopy may be needed to follow up on the findings. your doctor should have the results within two weeks from the time that the sample is received at the lab. cost: stool dna tests are more expensive than other stool based tests. if it’s not covered by insurance, the. The american cancer recommends that adults should begin colorectal cancer screening at age 50 using one of the following tests: flexible sigmoidoscopy (every five years), colonoscopy (every 10. Ct colonography must be done every 5 years. flexible sigmoidoscopy is not widely used for colorectal cancer screening in the u.s. it’s like a colonoscopy but looks at less than half of the colon and rectum. bowel prep is required before the test, but most people do not need sedation for this test. if polyps or suspicious areas are seen, a. A new colorectal screening test may be able to catch more cancers and high risk polyps than the currently available tests, new research found. the current stool tests don’t catch all polyps and.

Colorectal Cancer Screening Options Clinical Gastroenterology And Ct colonography must be done every 5 years. flexible sigmoidoscopy is not widely used for colorectal cancer screening in the u.s. it’s like a colonoscopy but looks at less than half of the colon and rectum. bowel prep is required before the test, but most people do not need sedation for this test. if polyps or suspicious areas are seen, a. A new colorectal screening test may be able to catch more cancers and high risk polyps than the currently available tests, new research found. the current stool tests don’t catch all polyps and.

Comments are closed.