Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry

Stoichiometry Limiting Excess Reactant Theoretical Percent Yield To calculate the corresponding mass of procaine, we use its structural formula (c13h20n2o2) to calculate its molar mass, which is 236.31 g mol. theoretical yield of procaine = 0.0729mol × 236.31g 1mol = 17.2g. c the actual yield was only 15.7 g of procaine, so the percent yield (via equation 4.3.5) is. Limiting reactant. percent yield. theoretical yield. 6.2: limiting reactant, theoretical yield, and percent yield is shared under a not declared license and was authored, remixed, and or curated by libretexts. when reactions are carried out using less than stoichiometric quantities of reactants, the amount of product generated will be.

Limiting Reactant Excess Reactant Theoretical Yield And Percent Yield This chemistry video tutorial shows you how to identify the limiting reagent and excess reactant. it shows you how to perform stoichiometric calculations an. Course: ap®︎ college chemistry > unit 4. lesson 4: stoichiometry. stoichiometry. worked example: calculating amounts of reactants and products. limiting reactant and reaction yields. worked example: calculating the amount of product formed from a limiting reactant. worked example: relating reaction stoichiometry and the ideal gas law. Identify the limiting reactant (s) and excess reactant (s). the limiting reactant is rb since it would yield the least amount of product (0.711 g mg). the excess reactant is mgcl 2 since its complete reaction would have yielded up to 0.878 g mg. calculate the mass of excess reactant that reacts. Excess reactant: reactant present in an amount greater than required by the reaction stoichiometry. limiting reactant: reactant present in an amount lower than required by the reaction stoichiometry, thus limiting the amount of product generated. percent yield: measure of the efficiency of a reaction, expressed as a percentage of the.

4 3 Limiting Reactant Theoretical Yield Percent Yield Youtube Identify the limiting reactant (s) and excess reactant (s). the limiting reactant is rb since it would yield the least amount of product (0.711 g mg). the excess reactant is mgcl 2 since its complete reaction would have yielded up to 0.878 g mg. calculate the mass of excess reactant that reacts. Excess reactant: reactant present in an amount greater than required by the reaction stoichiometry. limiting reactant: reactant present in an amount lower than required by the reaction stoichiometry, thus limiting the amount of product generated. percent yield: measure of the efficiency of a reaction, expressed as a percentage of the. The theoretical yield is 66.0 g hi. the percent yield would be calculated as: percent yield = actual yield theoretical yield × 100 62.3 g 66.0 g × 100 = 94.4 %. the percent yield is under 100% which is expected. experimental errors and side reactions can affect the percent yield. most chemists try to improve the percent yield of a reaction. 1. calculate the amount of the product (in moles or grams) formed from each reactant. 2. determine which reactant is limiting. 3. if necessary, calculate the amount of excess reactant required to react with the limiting reactant. then, subtract this amount from the starting quantity of the excess reactant.

Stoichiometry Limiting Reagent Reactant Percent Yield Practice The theoretical yield is 66.0 g hi. the percent yield would be calculated as: percent yield = actual yield theoretical yield × 100 62.3 g 66.0 g × 100 = 94.4 %. the percent yield is under 100% which is expected. experimental errors and side reactions can affect the percent yield. most chemists try to improve the percent yield of a reaction. 1. calculate the amount of the product (in moles or grams) formed from each reactant. 2. determine which reactant is limiting. 3. if necessary, calculate the amount of excess reactant required to react with the limiting reactant. then, subtract this amount from the starting quantity of the excess reactant.
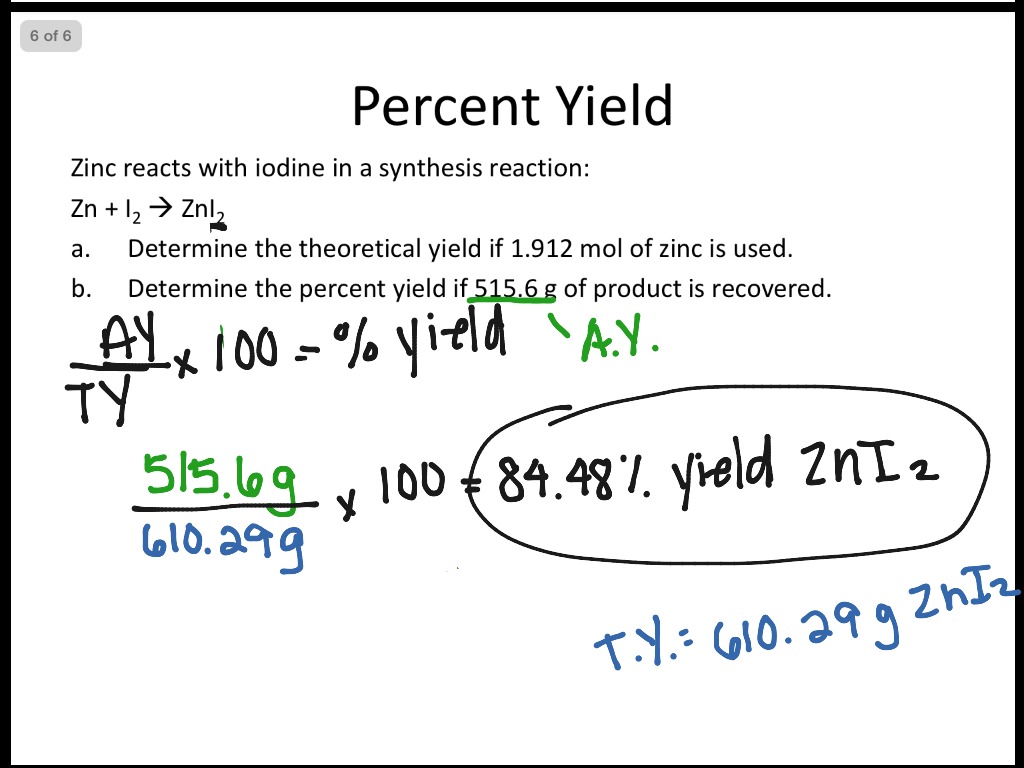
Limiting Reactant And Percent Yield Science Chemistry Stoichiometry

Comments are closed.