Solved Oints Figure 1 Shows A Carnot Heat Pump Cycle Chegg

Solved Oints Figure 1 Shows A Carnot Heat Pump Cycle Chegg Question: oints figure 1 shows a carnot heat pump cycle operating at steady state with water as the working fluid. the condenser temperature is 200°c, with saturated vapor entering and saturated liquid exiting. the evaporator temperature is 20°c. . determine the rate of heat transfer and work for each process, in kj kg, of water flowing 2. Question: figure p6.169 shows a carnot heat pump cycle operating at steady state with ammonia as the working fluid. the condenser temperature is 120of, with saturated vapor entering and saturated liquid exiting. the evaporator temperature is 10of. (a) determine the heat transfer and work for each process, in btu per lb of ammonia flowing.

Solved Oints Figure 1 Shows A Carnot Heat Pump Cycle Chegg The figure below shows a carnot heat pump cycle operating at steady state with 1 kg s of ammonia as the working fluid. the condenser temperature is 120 f, with saturated vapor entering and saturated liquid exiting. the evaporator temperature is 10 f. recall that a carnot cycle is composed of alternating adiabatic and isothermal processes, and. A carnot heat pump imagine a carnot heat pump operates between an outside temperature of 0 °c 0 °c and an inside temperature of 20.0 °c 20.0 °c. what is the work needed if the heat delivered to the inside of the house is 30.0 kj? strategy because the heat pump is assumed to be a carnot pump, its performance coefficient is given by k p = q h. A carnot heat pump (or carnot refrigerator) is a reverse carnot heat engine, that absorbs heat from a cold thermal reservoir and transfers it to a warmer thermal reservoir. as we will show below, it is the most efficient heat pump operating between two given temperatures. first, we will determine the coefficient of performance of the carnot. Figure p6.5 shows a carnot heat pump cycle operating at steady state with ammonia as the working fluid. the condenser temperature is 120°f, with saturated vapor entering and saturated liquid exiting. the evaporator temperature is 10°f. (a) determine the heat transfer and work for each process in btu per lb of ammonia flowing.
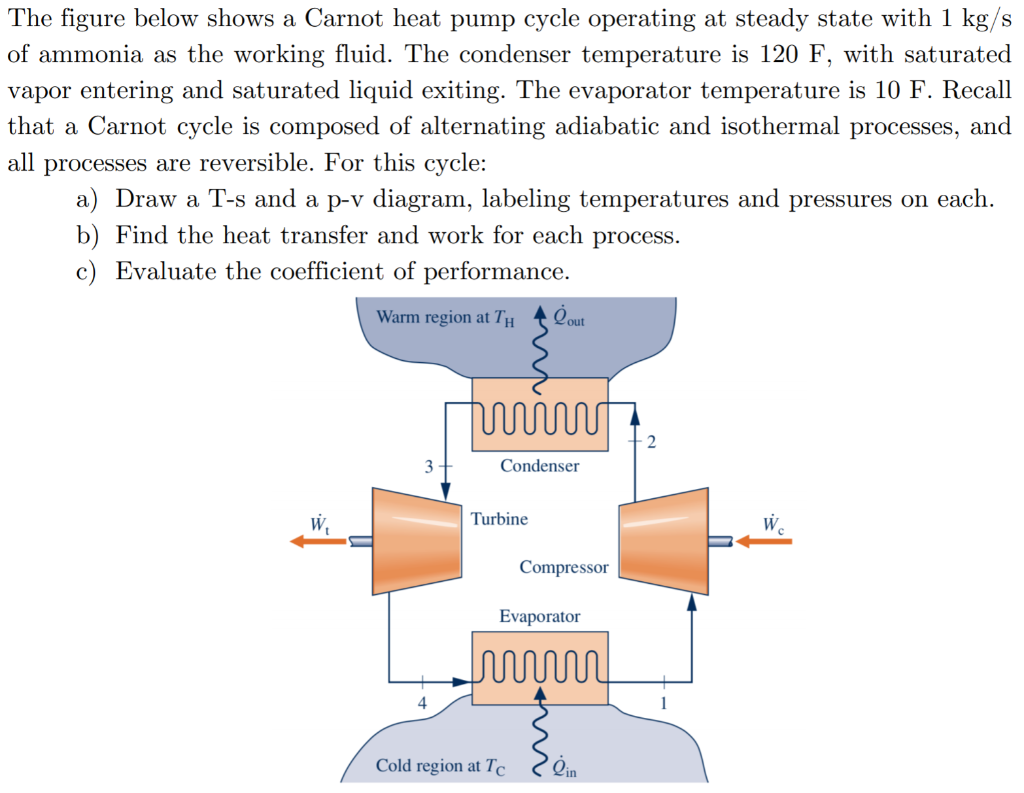
Solved The Figure Below Shows A Carnot Heat Pump Cycle Chegg A carnot heat pump (or carnot refrigerator) is a reverse carnot heat engine, that absorbs heat from a cold thermal reservoir and transfers it to a warmer thermal reservoir. as we will show below, it is the most efficient heat pump operating between two given temperatures. first, we will determine the coefficient of performance of the carnot. Figure p6.5 shows a carnot heat pump cycle operating at steady state with ammonia as the working fluid. the condenser temperature is 120°f, with saturated vapor entering and saturated liquid exiting. the evaporator temperature is 10°f. (a) determine the heat transfer and work for each process in btu per lb of ammonia flowing. The standard of comparison for refrigeration cycles is the reversed carnot cycle. a refrigerator or heat pump that operates on the reversed carnot cycle is called a carnot refrigerator or a carnot heat pump, and their cops are cop tt t tt cop tt t tt r carnot hl l hl hp carnot lh h hl,, = − = − = − = − 1 1 1 1. 3. the work done on the gas in one cycle of the carnot refrigerator is shown and given by the area enclosed by the loop mponm. the work done on the ideal gas is equal to the area enclosed by the path of the pv diagram. from the first law, this work is given by. w = qh −qc. (4.6.13) (4.6.13) w = q h − q c.

Solved The Figure Below Shows A Carnot Heat Pump At Which Chegg The standard of comparison for refrigeration cycles is the reversed carnot cycle. a refrigerator or heat pump that operates on the reversed carnot cycle is called a carnot refrigerator or a carnot heat pump, and their cops are cop tt t tt cop tt t tt r carnot hl l hl hp carnot lh h hl,, = − = − = − = − 1 1 1 1. 3. the work done on the gas in one cycle of the carnot refrigerator is shown and given by the area enclosed by the loop mponm. the work done on the ideal gas is equal to the area enclosed by the path of the pv diagram. from the first law, this work is given by. w = qh −qc. (4.6.13) (4.6.13) w = q h − q c.

Solved Carnot Heat Pump Is An Ideal Cycle As Shown In Chegg

Comments are closed.