Solved Hw 4 1 Consider A Carnot Cycle Executed In A Closed Chegg
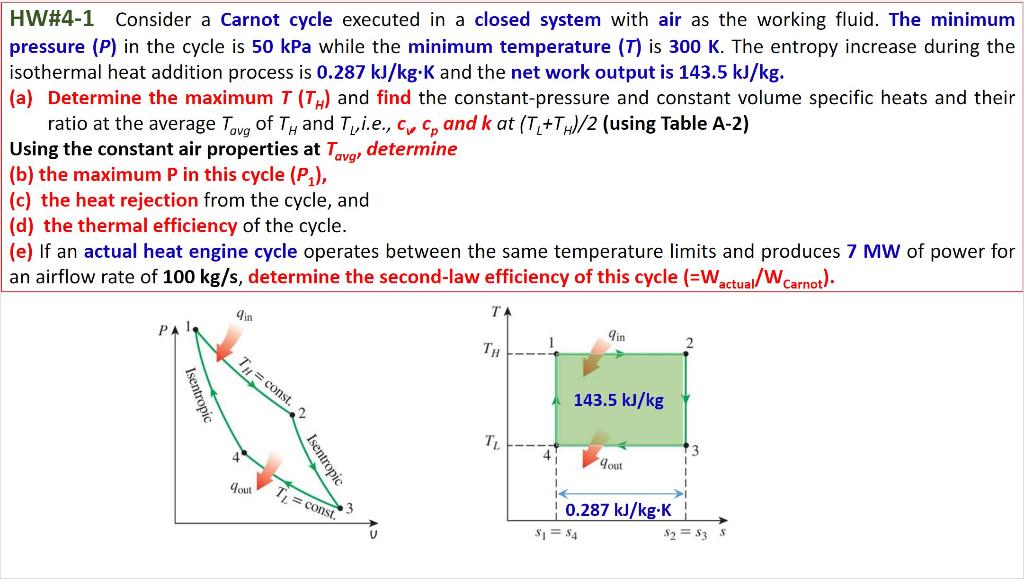
Solved Hw 4 1 Consider A Carnot Cycle Executed In A Closed Chegg Hw#4 1 consider a carnot cycle executed in a closed system with air as the working fluid. the minimum pressure (p) in the cycle is 50 kpa while the minimum temperature (t) is 300 k. the entropy increase during the isothermal heat addition process is 0.287 kj kg.k and the net work output is 143.5 kj kg. (a) determine the maximum t (th) and find the. There are 4 steps to solve this one. the carnot cycle, whose all processes are reversible and is a hypothetical cycle. consider a carnot cycle executed in a closed system with air as the working fluid. the maximum pressure in the cycle is 1300kpa while the maximum temperature is 950 k. if the entropy increase during the isothermal heat.

Solved Consider A Carnot Cycle Executed In A Closed System Chegg Consider a carnot cycle executed in a closed system with air as the working fluid. the maximum pressure in the cycle is 1300 kpa, while the maximum temperature is 950 k. the entropy increase during the isothermal heat addition process is 0.2 kj kg ⋅ k and the net work output is 110 kj kg. Chapter 9: problem 20. consider a carnot cycle executed in a closed system with 0.6 kg of air. the temperature limits of the cycle are 300 and 1100 k, and the minimum and maximum pressures that occur during the cycle are 20 and 3000 kpa. assuming constant specific heats, determine the net work output per cycle. Chapter 9: problem 21. consider a carnot cycle executed in a closed system with air as the working fluid. the maximum pressure in the cycle is 1300 kpa while the maximum temperature is 950 k if the entropy increase during the isothermal heat rejection process is 0.25 kj kg ⋅ k and the net work output is 100 kj kg determine (a) the minimum. A carnot cycle is executed in a closed system and uses $0.0025 \mathrm{~kg}$ of air as the working fluid. the cycle efficiency is 60 percent, and the lowest temperature in the cycle is $300 \mathrm{~k}$. the pressure at the beginning of the isentropic expansion is $700 \mathrm{kpa},$ and at the end of the isentropic compression it is 1 mpa.

Solved Consider A Carnot Cycle Executed In A Closed System Chegg Chapter 9: problem 21. consider a carnot cycle executed in a closed system with air as the working fluid. the maximum pressure in the cycle is 1300 kpa while the maximum temperature is 950 k if the entropy increase during the isothermal heat rejection process is 0.25 kj kg ⋅ k and the net work output is 100 kj kg determine (a) the minimum. A carnot cycle is executed in a closed system and uses $0.0025 \mathrm{~kg}$ of air as the working fluid. the cycle efficiency is 60 percent, and the lowest temperature in the cycle is $300 \mathrm{~k}$. the pressure at the beginning of the isentropic expansion is $700 \mathrm{kpa},$ and at the end of the isentropic compression it is 1 mpa. Answered step by step. consider a carnot cycle executed in a closed system with 0.6 kg 0.6 k g of air. the temperature limits of the cycle are 300 and 1100 k 1100 k, and the minimum and maximum pressures that occur during the cycle are 20 and 3000kpa 3000 k p a. assuming constant specific heats, determine the net work output per cycle. Question: consider a carnot refrigeration cycle executed in a closed system in the saturated liquid vapor mixture region using 0.96 kg of refrigerant 134a as the working fluid. it is known that the maximum absolute temperature in the cycle is 1.2 times the minimum absolute temperature, and the net work input to the cycle is 22 kj.

Comments are closed.