Solved Consider An Ideal Carnot Heat Pump Cycle That Uses Chegg

Solved Consider An Ideal Carnot Heat Pump Cycle That Uses Chegg Mechanical engineering questions and answers. consider an ideal carnot heat pump cycle that uses water and operates between a cold and a hot reservoir at temperatures of 120∘c and 200∘c respectively. the processes involved are: process a, from state 1 to state 2: adiabatic compression. process b, from state 2 to state 3: isothermal phase. Consider an ideal saturated steam rankine cycle with single stage regeneration, operating between 1000 psia and 1.0 psia. the regeneration process uses an open feedwater heater where some steam is extracted from the turbine for preheating the feedwater before entering the boiler. neglecting pump work, calculate (a) the condition at the.

Solved Consider An Ideal Carnot Heat Pump Cycle That Uses Chegg The cycle of an ideal gas carnot refrigerator is represented by the pv diagram of figure 4.13. it is a carnot engine operating in reverse. it is a carnot engine operating in reverse. the refrigerator extracts heat q c q c from a cold temperature reservoir at t c t c when the ideal gas expands isothermally. The second law of thermodynamics can be restated in terms of the carnot cycle, and so what carnot actually discovered was this fundamental law. any heat engine employing the carnot cycle is called a carnot engine. what is crucial to the carnot cycle—and, in fact, defines it—is that only reversible processes are used. 11.5 a supply of geothermal hot water is to be used as the energy source in an ideal rankine cycle, with r 134a as the cycle working fluid. saturated vapor r 134a leaves the boiler at a temperature of 85°c, and the condenser temperature is 40°c. calculate the thermal efficiency of this cycle. cv: pump (use r 134a table b.5) w p = h 2 h 1. 3. the work done on the gas in one cycle of the carnot refrigerator is shown and given by the area enclosed by the loop mponm. the work done on the ideal gas is equal to the area enclosed by the path of the pv diagram. from the first law, this work is given by. w = qh −qc. (4.6.13) (4.6.13) w = q h − q c.

Solved Consider An Ideal Carnot Heat Pump Cycle That Uses Chegg 11.5 a supply of geothermal hot water is to be used as the energy source in an ideal rankine cycle, with r 134a as the cycle working fluid. saturated vapor r 134a leaves the boiler at a temperature of 85°c, and the condenser temperature is 40°c. calculate the thermal efficiency of this cycle. cv: pump (use r 134a table b.5) w p = h 2 h 1. 3. the work done on the gas in one cycle of the carnot refrigerator is shown and given by the area enclosed by the loop mponm. the work done on the ideal gas is equal to the area enclosed by the path of the pv diagram. from the first law, this work is given by. w = qh −qc. (4.6.13) (4.6.13) w = q h − q c. Greater than that of the carnot engine. consider figure 22.9, which shows the hypothetical engine with e 7 e c on the left connected between hot and cold res ervoirs. in addition, let us attach a carnot engine between the same reservoirs. because the carnot cycle is reversible, the carnot engine can be run in reverse as a carnot heat pump as. Volume in the otto cycle). by contrast, a carnot engine, by definition is a reversible engine operating be tween only two heat reservoirs: all processes are either isothermal (i.e., heat transfer at constant temperature) or adiabatic (i.e., no heat transfer). later, we will see a carnot cycle for an ideal gas on a p v diagram.
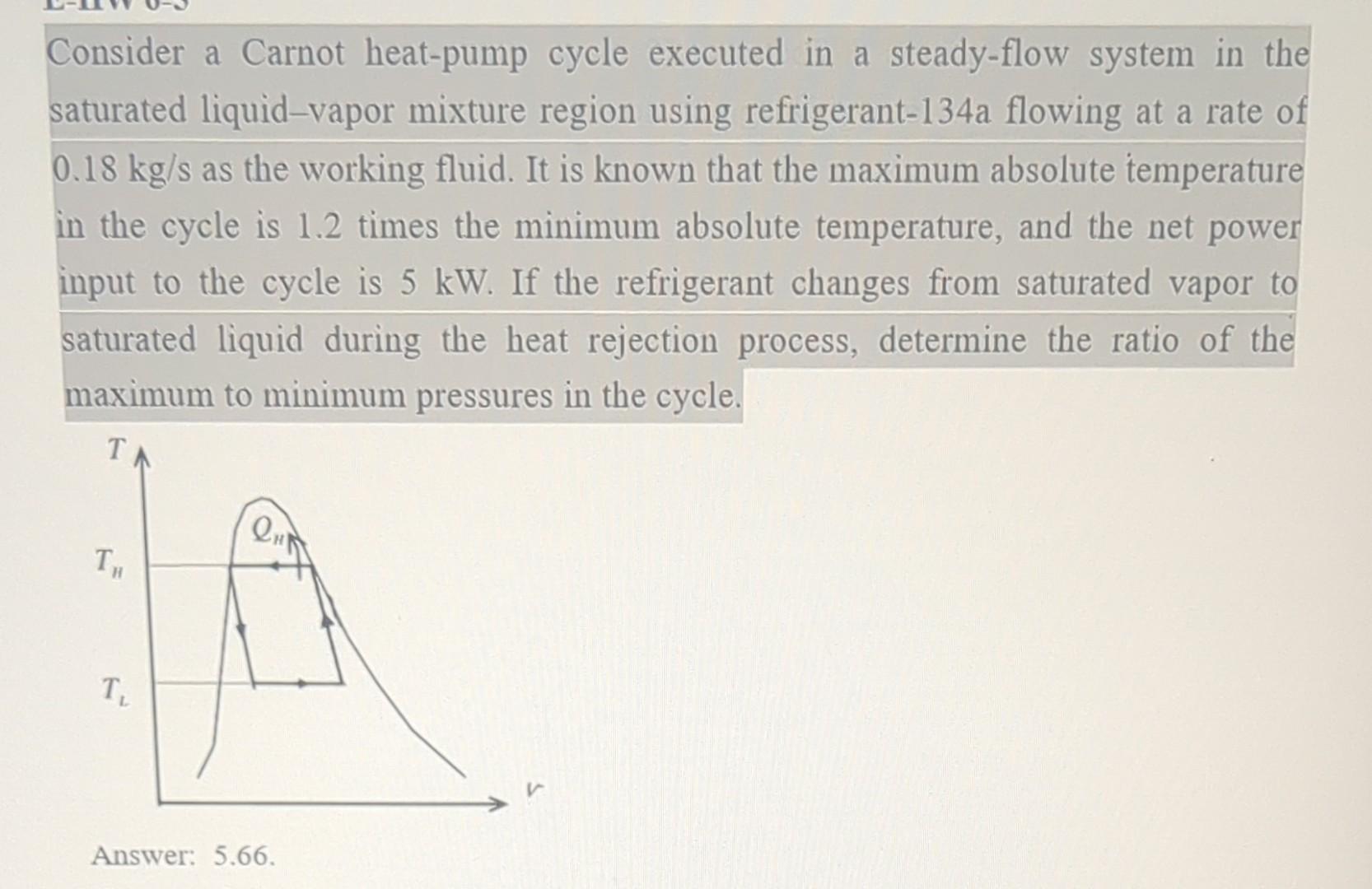
Solved Consider A Carnot Heat Pump Cycle Executed In A Chegg Greater than that of the carnot engine. consider figure 22.9, which shows the hypothetical engine with e 7 e c on the left connected between hot and cold res ervoirs. in addition, let us attach a carnot engine between the same reservoirs. because the carnot cycle is reversible, the carnot engine can be run in reverse as a carnot heat pump as. Volume in the otto cycle). by contrast, a carnot engine, by definition is a reversible engine operating be tween only two heat reservoirs: all processes are either isothermal (i.e., heat transfer at constant temperature) or adiabatic (i.e., no heat transfer). later, we will see a carnot cycle for an ideal gas on a p v diagram.

Solved Consider An Ideal Carnot Heat Pump Cycle That Uses Chegg
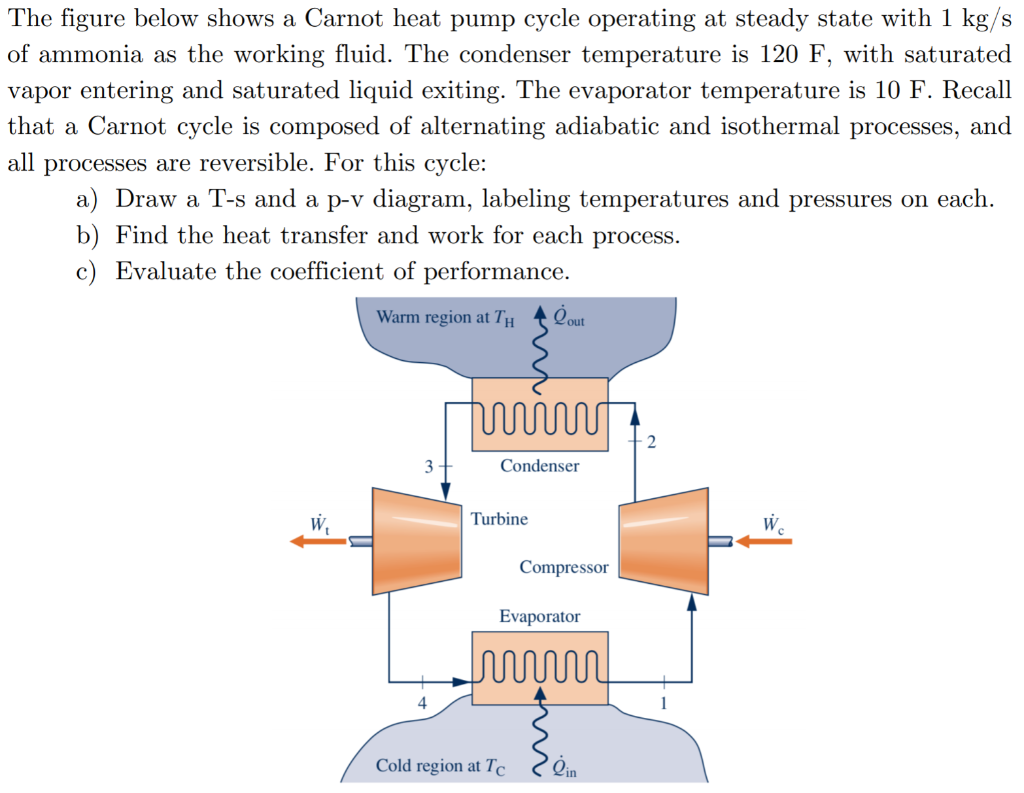
Solved The Figure Below Shows A Carnot Heat Pump Cycle Chegg

Comments are closed.