Ppt Colligative Properties Of Solutions Powerpoint Presentation Free

Ppt Colligative Properties Of Solutions Powerpoint Presentation Free There are four main colligative properties: vapor pressure lowering, freezing point depression, boiling point elevation, and osmotic pressure. each property is directly proportional to the molality of the solution and can be calculated using the appropriate constant for the solvent. ideal & non ideal solutions, comparison between ideal & non. Download now. colligative properties such as boiling point elevation, freezing point depression, and osmotic pressure depend only on the number of solute particles in solution, not their identity. the document discusses these properties and how they are affected when solutes dissolve. it explains that boiling point is elevated and freezing.

Ppt Colligative Properties Of Solutions Powerpoint Presentation Free Colligative properties of solutions. step 1 breaking up the solute into individual components (expanding the solute) step 2 overcoming the intermolecular forces in the solvent to make room for the solute (expanding the solvent) step 3. download presentation. hypotonic solution. boiling point freezing point. as low. long hydrophobic tail. po vapor. The document discusses colligative properties, which are properties of solutions that depend on the number of solute particles present. there are four main colligative properties: vapor pressure lowering, freezing point depression, boiling point elevation, and osmotic pressure. each property is directly proportional to the molality of the. Colligative properties • colligative property: a property that depends only upon the number of solute particles (concentration), and not upon their identity. • three important colligative properties of solutions. • vapor pressure lowering • boiling point elevation • freezing point depression. Presentation transcript. section2 colligative properties of solutions chapter 13 colligative properties of solutions • properties that depend on the concentration of solute particles but not on their identity are called colligative properties. • vapor pressure lowering • freezing point depression • boiling point elevation • osmotic.
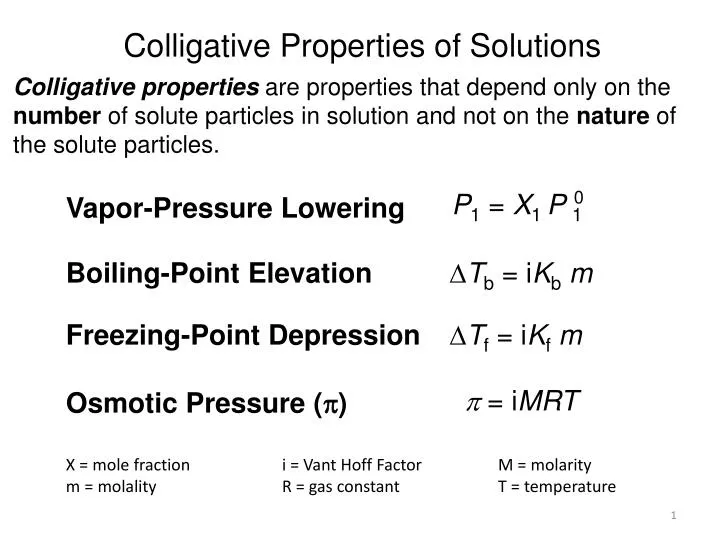
Ppt Colligative Properties Of Solutions Powerpoint Presentation Free Colligative properties • colligative property: a property that depends only upon the number of solute particles (concentration), and not upon their identity. • three important colligative properties of solutions. • vapor pressure lowering • boiling point elevation • freezing point depression. Presentation transcript. section2 colligative properties of solutions chapter 13 colligative properties of solutions • properties that depend on the concentration of solute particles but not on their identity are called colligative properties. • vapor pressure lowering • freezing point depression • boiling point elevation • osmotic. 2. colligative properties. depend only on the number of solute particles in. a solution. does not depend on the identity of particles. 3. boiling point elevation. a nonvolatile solute lowers the vapor pressure. a higher t is required to reach the 1 atm of. Physical properties of solutions differ from the solvent they were made from differences are due to the number of solute particles in solution (not the type of solute) these differences in physical properties are called colligative properties colligative properties include: vapor pressure freezing point boiling point osmotic pressure. 3 1.

Ppt Colligative Properties Of Solutions Powerpoint Presentation Free 2. colligative properties. depend only on the number of solute particles in. a solution. does not depend on the identity of particles. 3. boiling point elevation. a nonvolatile solute lowers the vapor pressure. a higher t is required to reach the 1 atm of. Physical properties of solutions differ from the solvent they were made from differences are due to the number of solute particles in solution (not the type of solute) these differences in physical properties are called colligative properties colligative properties include: vapor pressure freezing point boiling point osmotic pressure. 3 1.

Ppt Colligative Properties In Solution Powerpoint Presentation Free

Comments are closed.