Pharmaceutics Free Full Text Quality By Design As A Tool In The
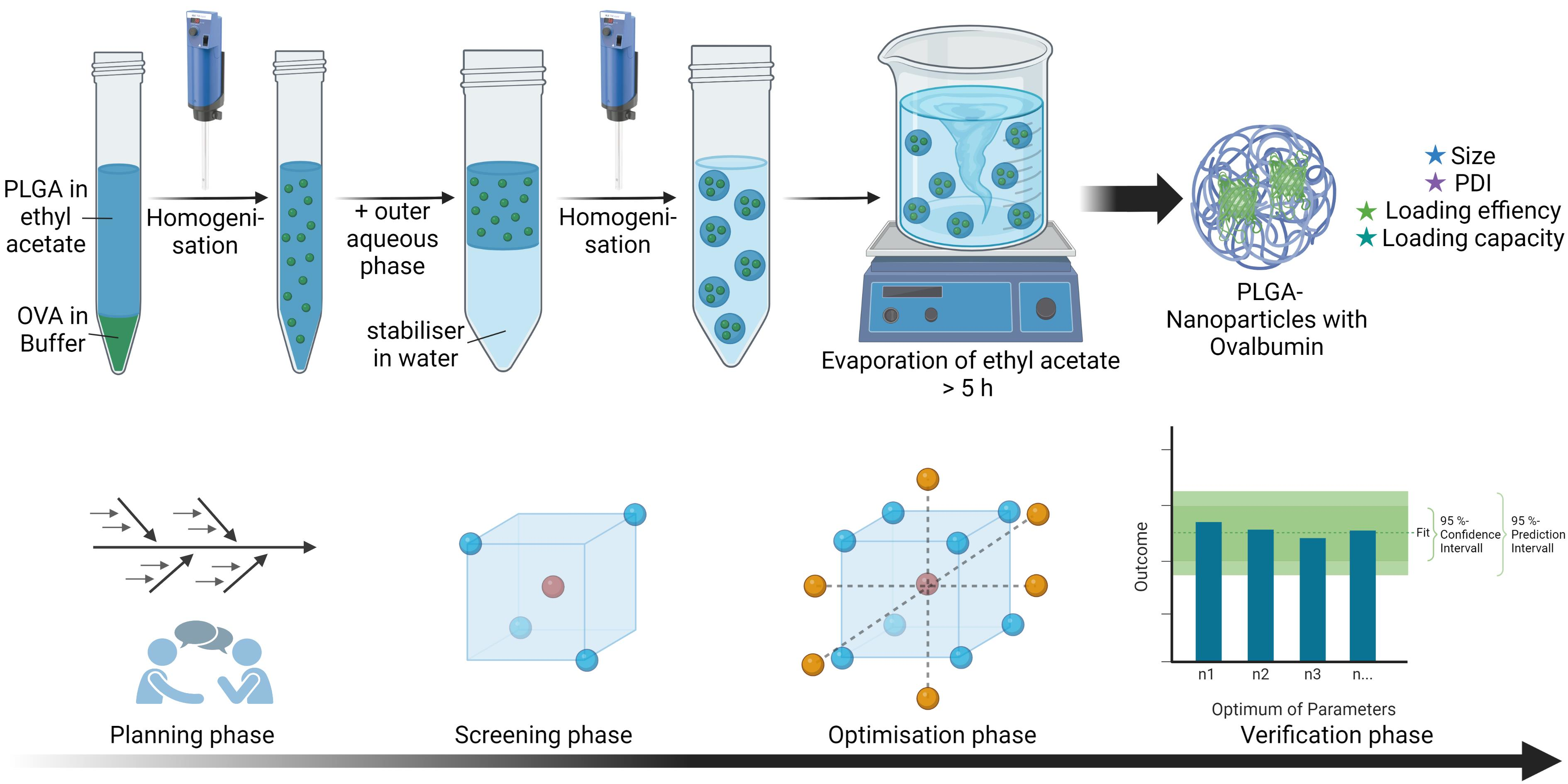
Pharmaceutics Free Full Text Quality By Design As A Tool In The Search text. search type . add circle outline r. quality by design as a tool in the optimisation of nanoparticle preparation—a case study of plga nanoparticles. Pharmaceutical industry. qbd can be considered to be system based approach to the design, development, and delivery of any product or service to a consumer. it is an approach to pharmaceutical development that begins with predefined objectives and emphasizes product and process understanding and process control. process parameters and quality attributes are identified for each unit operation.
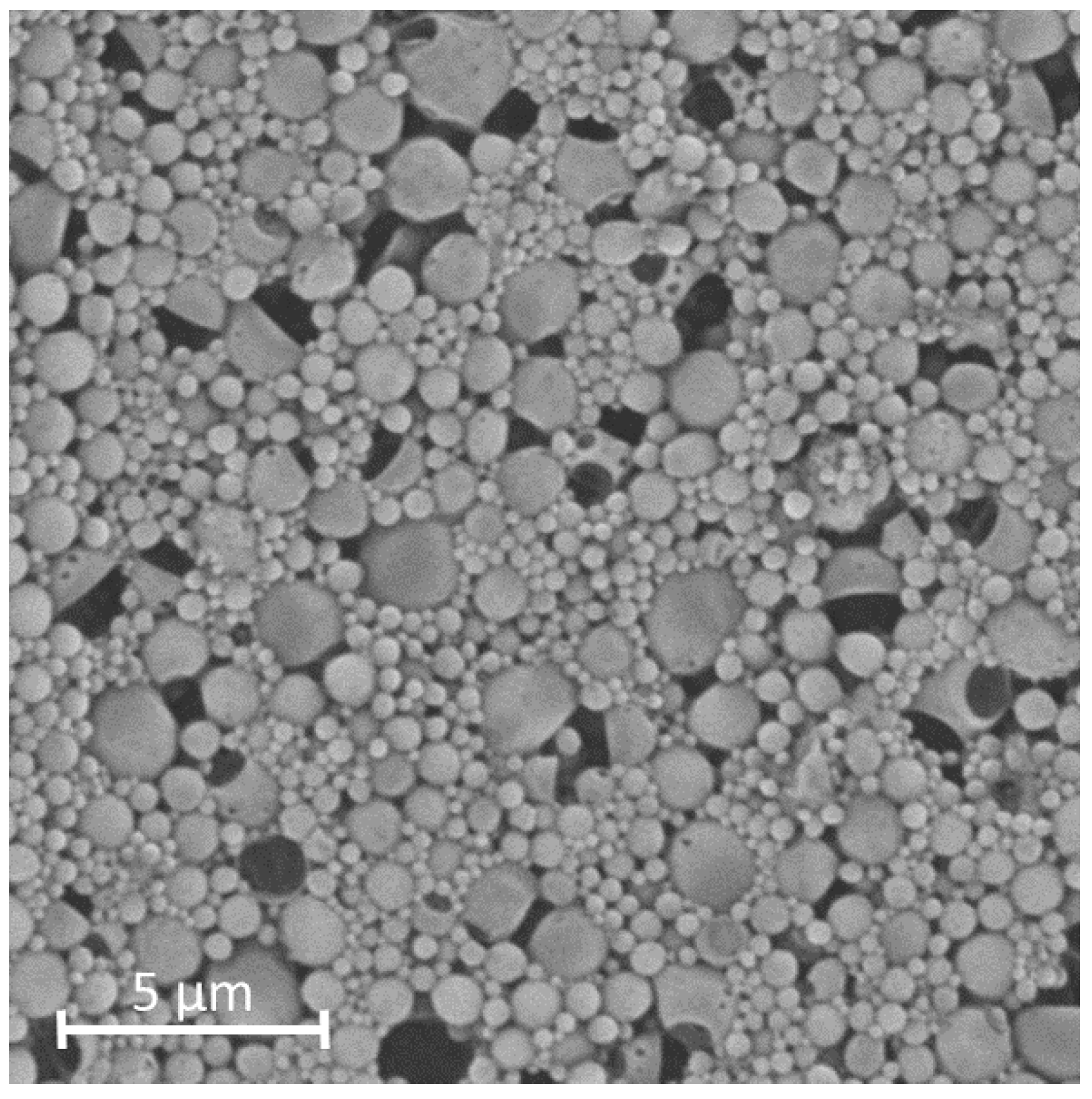
Pharmaceutics Free Full Text Quality By Design As A Tool In The Abstract. quality by design (qbd) represents a transformative approach to pharmaceutical development, emphasizing a systematic and science driven methodology to ensure consistent product quality. 4.7.1 quality risk assessment (qra) 83 4.8 design of experiments (doe) 87 4.9 critical process parameters (cpps) 88 4.10 design space 88 4.11 control strategy 89 4.12 references 91 5 the role of excipients in quality by design (qbd) 97 brian carlin 5.1 introduction 97 5.2 quality of design (qbd) 98 5.3 design of experiments (doe) 100. This review further clarifies the concept of pharmaceutical quality by design (qbd) and describes its objectives. qbd elements include the following: (1) a quality target product profile (qtpp) that identifies the critical quality attributes (cqas) of the drug product; (2) product design and understanding including identification of critical material attributes (cmas); (3) process design and. Quality by design (qbd) is a systematic approach to drug development, which begins with predefined objectives, and uses science and risk management approaches to gain product and process understanding and ultimately process control. the concept of qbd can be extended to analytical methods. qbd mandates the definition of a goal for the method, and emphasizes thorough evaluation and scouting of.
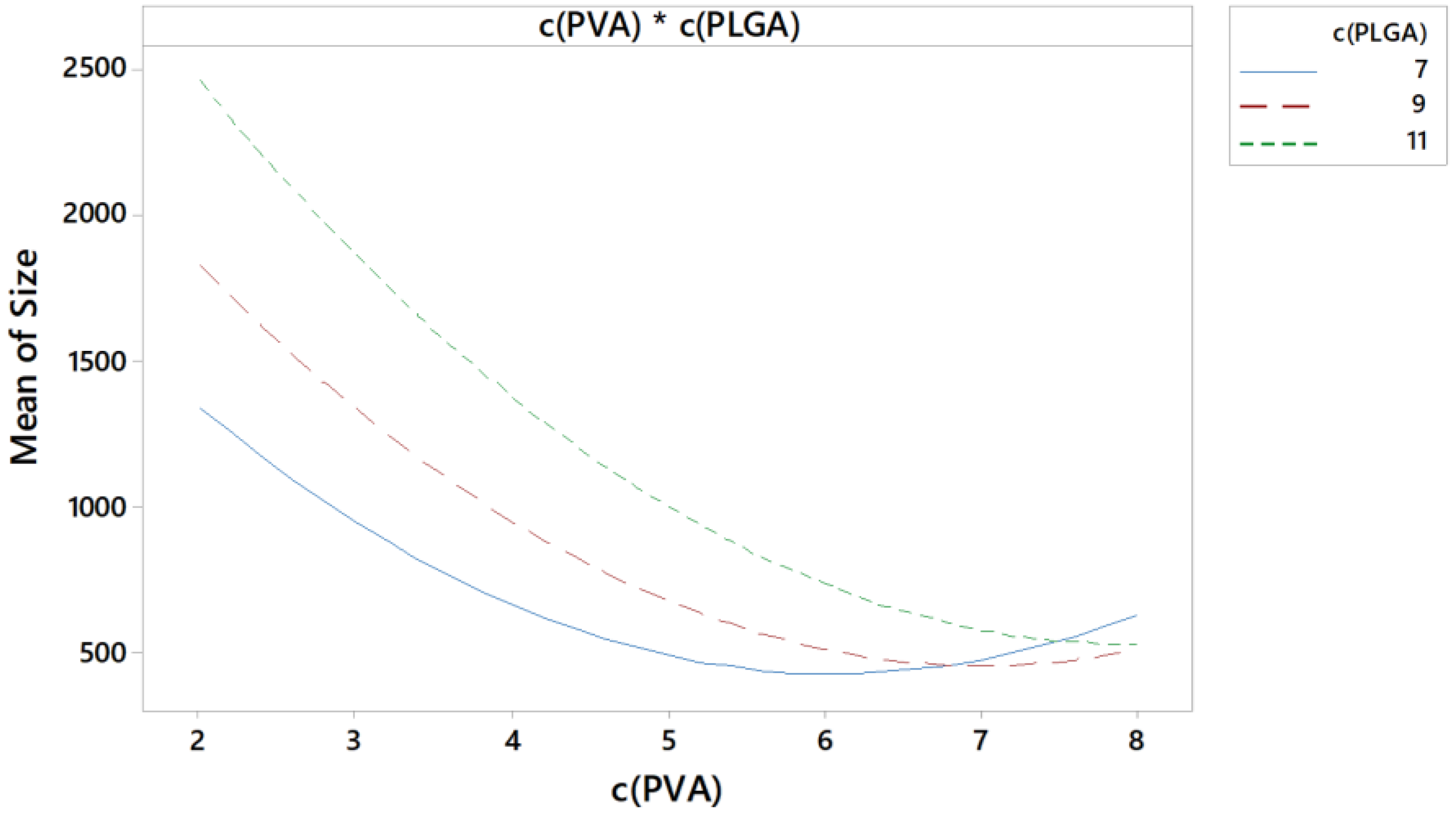
Pharmaceutics Free Full Text Quality By Design As A Tool In The This review further clarifies the concept of pharmaceutical quality by design (qbd) and describes its objectives. qbd elements include the following: (1) a quality target product profile (qtpp) that identifies the critical quality attributes (cqas) of the drug product; (2) product design and understanding including identification of critical material attributes (cmas); (3) process design and. Quality by design (qbd) is a systematic approach to drug development, which begins with predefined objectives, and uses science and risk management approaches to gain product and process understanding and ultimately process control. the concept of qbd can be extended to analytical methods. qbd mandates the definition of a goal for the method, and emphasizes thorough evaluation and scouting of. Quality by design (qbd) design space. control strategy. 1. introduction. the pharmaceutical market has been considered as one of the highly regulated sectors, which has been continuously providing quality drug products for human use to provide desired pharmacotherapeutic effects for the treatment of diverse ailments. Request full text pdf. the application of quality by design (qbd) in pharmaceutical product development is now a thrust area for the regulatory authorities and the pharmaceutical industry.
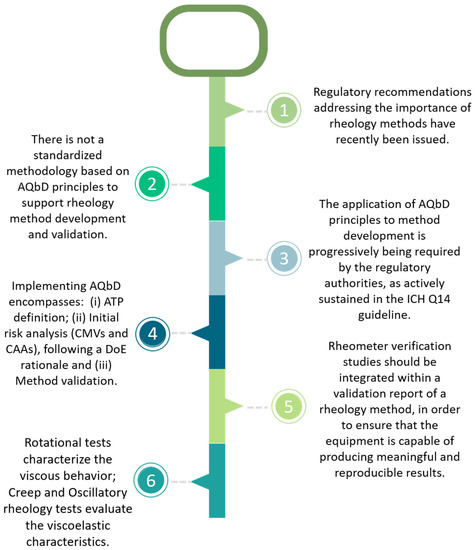
Pharmaceutics Free Full Text Rheology Of Complex Topical Quality by design (qbd) design space. control strategy. 1. introduction. the pharmaceutical market has been considered as one of the highly regulated sectors, which has been continuously providing quality drug products for human use to provide desired pharmacotherapeutic effects for the treatment of diverse ailments. Request full text pdf. the application of quality by design (qbd) in pharmaceutical product development is now a thrust area for the regulatory authorities and the pharmaceutical industry.

Comments are closed.