Ph Indicators Explained
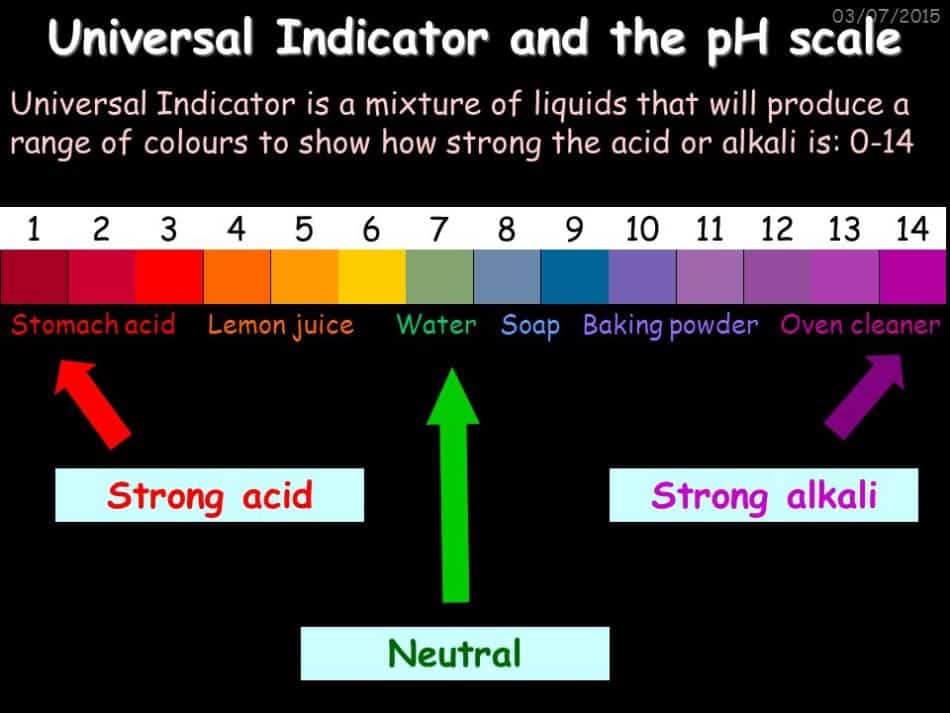
Universal Indicator And The Ph Scale Mywaterearth Sky Learn what a ph indicator is, how it works, and what it looks like in different ph solutions. find out how to use ph indicators for measuring and titrating acid base reactions. Ph ≈ −log[h3o ] (3) (3) p h ≈ − l o g [h 3 o ] a ph of 7 indicates a neutral solution like water. a ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. ultimately, the ph value indicates how much h has dissociated from molecules within a solution. the lower the ph value, the higher.
Defining Ph Indicator And Understanding Its Importance In Life A ph indicator is a halochromic chemical compound added in small amounts to a solution so the ph (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and or emission properties. [1] hence, a ph indicator is a chemical detector for hydronium ions (h 3 o ) or hydrogen ions (h ) in the. A universal indicator is a mixture of several indicators that can provide a continuous color change over a range of ph values, typically from about ph 2 to ph 10. universal indicator paper is made from absorbent paper that has been impregnated with universal indicator. Learn how to use ph indicators to measure the acidity or basicity of a solution by their color change. see a chart of common ph indicators, their ph ranges, and their solutions. An indicator is usually some weak organic acid or base dye that changes colors at definite ph values. the weak acid form (hin) will have one color and the weak acid negative ion (in ) will have a different color. the weak acid equilibrium is: hin → h in . for phenolphthalein: ph 8.2 = colorless; ph 10 = red.
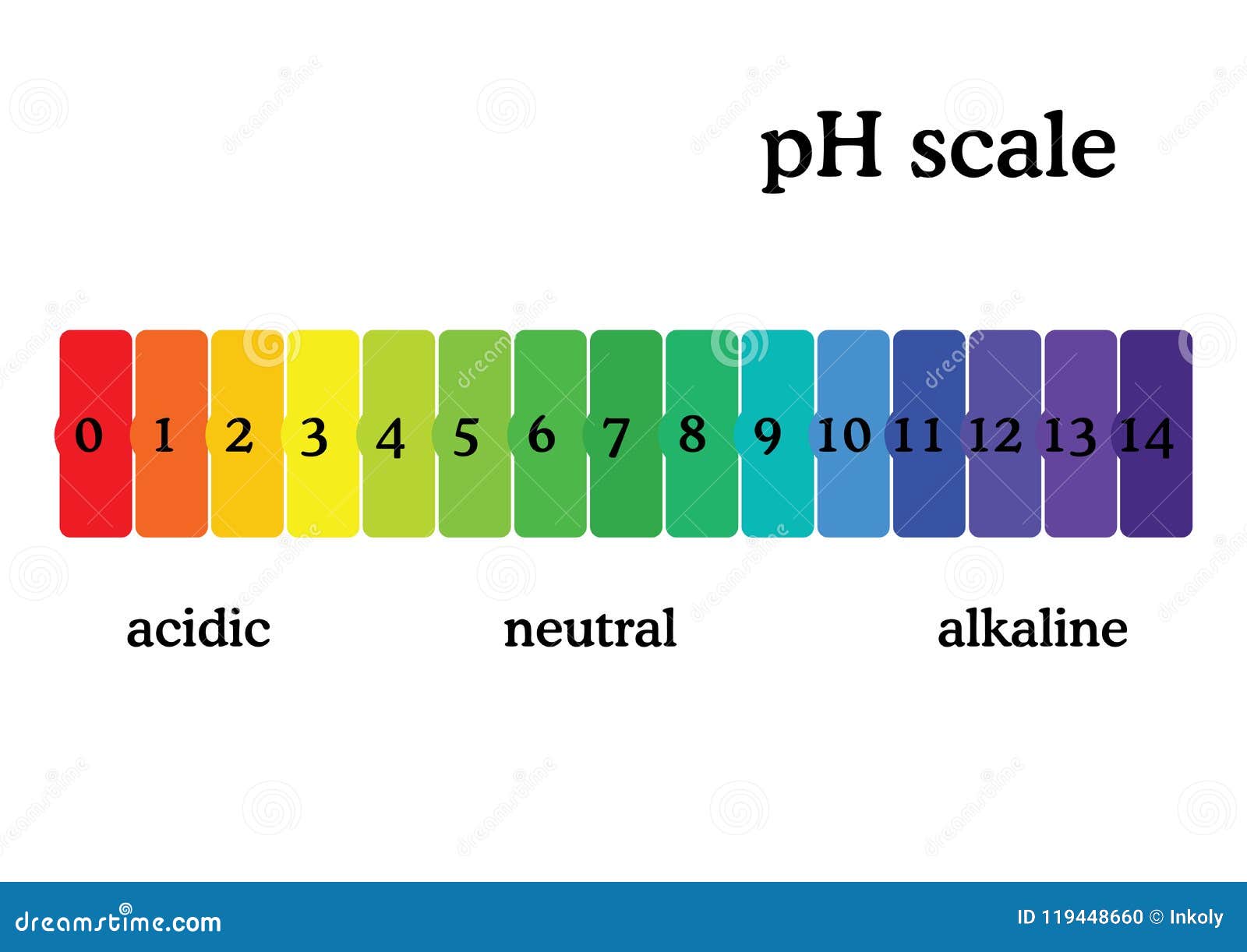
Ph Scale Diagram With Corresponding Acidic Or Alcaline Values Learn how to use ph indicators to measure the acidity or basicity of a solution by their color change. see a chart of common ph indicators, their ph ranges, and their solutions. An indicator is usually some weak organic acid or base dye that changes colors at definite ph values. the weak acid form (hin) will have one color and the weak acid negative ion (in ) will have a different color. the weak acid equilibrium is: hin → h in . for phenolphthalein: ph 8.2 = colorless; ph 10 = red. Ph and indicators. this page introduces the ph scale to measure the degree of acidity or alkalinity of a solution. it also introduces some common ways to test whether a solution is acidic or alkaline. the ph scale. the ph scale is used to measure the acidity or alkalinity of a solution. the key points are: a ph of 7 shows the solution to be. Ph measures the acidity or alkalinity of a substance. ph indicators are substances that change color in response to different ph levels. real world examples of ph include lemon juice (acidic) and baking soda (alkaline). the ph scale ranges from 0 14, with 0 being highly acidic and 14 being highly alkaline. ph measurement is essential in various.
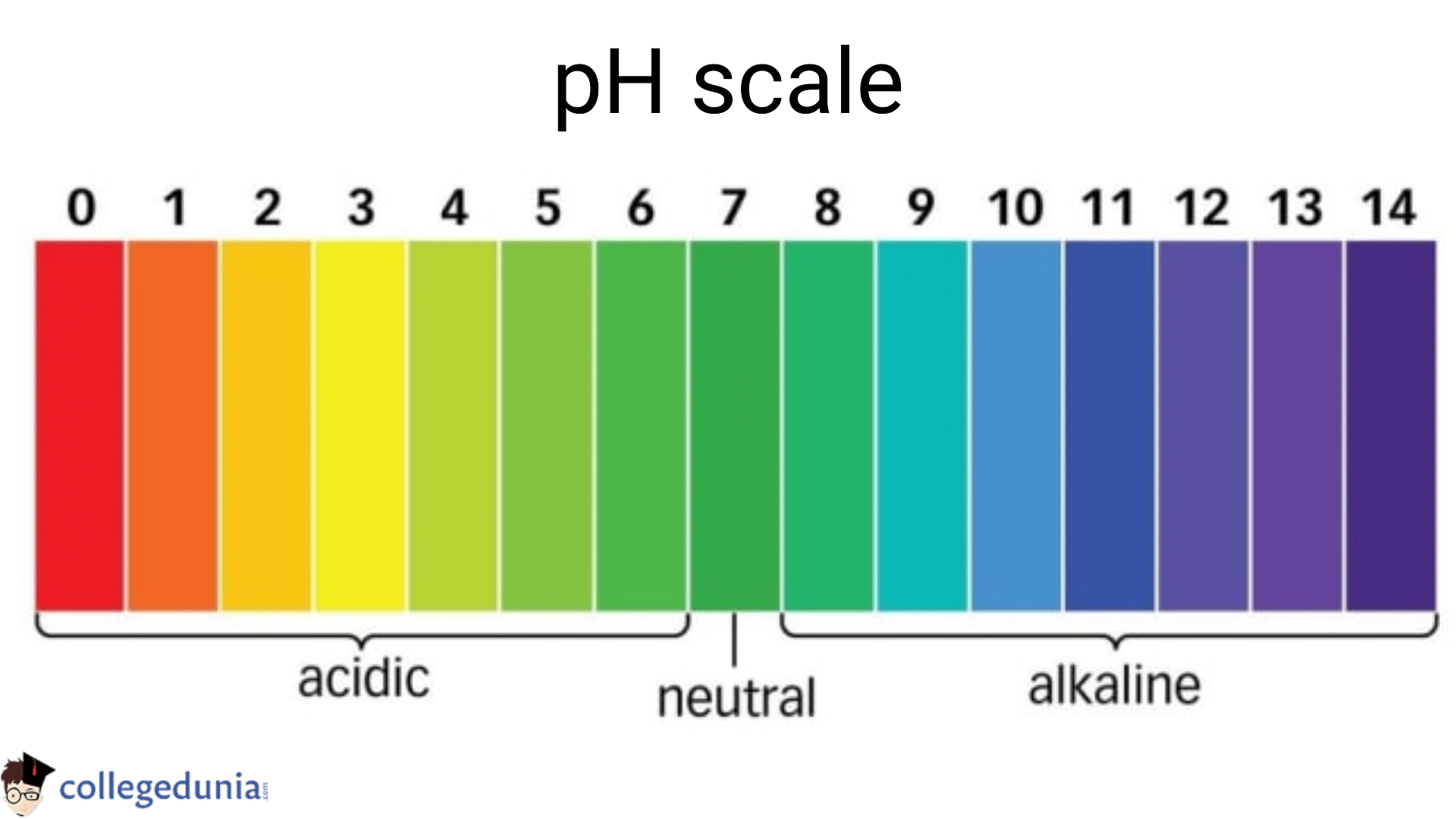
Ph Scale Diagram Ph and indicators. this page introduces the ph scale to measure the degree of acidity or alkalinity of a solution. it also introduces some common ways to test whether a solution is acidic or alkaline. the ph scale. the ph scale is used to measure the acidity or alkalinity of a solution. the key points are: a ph of 7 shows the solution to be. Ph measures the acidity or alkalinity of a substance. ph indicators are substances that change color in response to different ph levels. real world examples of ph include lemon juice (acidic) and baking soda (alkaline). the ph scale ranges from 0 14, with 0 being highly acidic and 14 being highly alkaline. ph measurement is essential in various.

Comments are closed.