One Of The Structural Isomers Of C_3 H_6 Os Is The Compound That Mak
31 Show Structural Isomers In C3h6o One of the structural isomers of c 3 h 6 os is the compound that makes you cry when you slice onions. write lewis structures for two isomers of this molecule. In structural isomerism, the atoms are arranged in a completely different order. structural isomers could be chain isomers, position isomers, and functional group isomers. the difference is easier to see with specific examples. what follows looks at some of the ways that structural isomers can arise. the names of the various forms of structural.
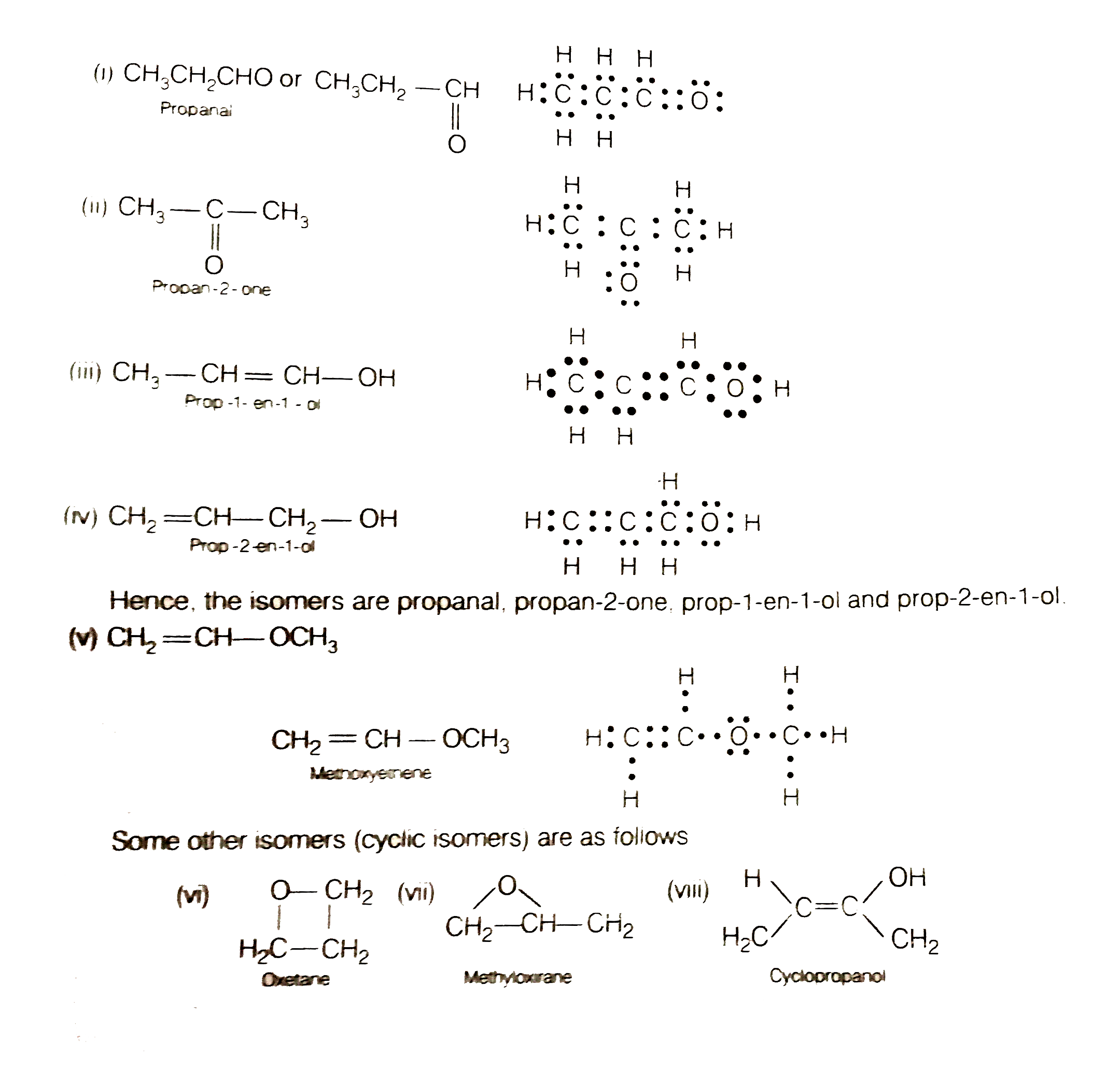
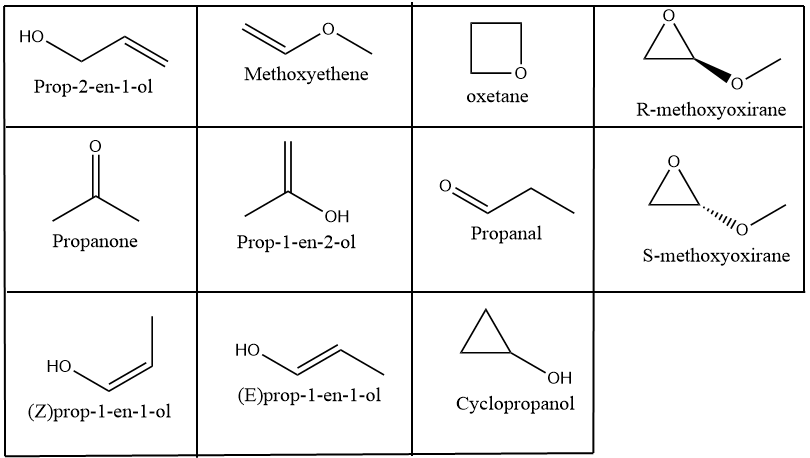
Draw The Possible Isomers Of The Compound With Molecular Formula C 3 H Which isomers can be drawn for c 3 h 6 o. by observing the molecular formula c 3 h 6 o, there are three elements; carbon, hydrogen and oxygen. because oxygen is included as an element, we can try to draw following organic compound types (only one oxygen atom is included) if possible. ether; alcohols. Constitutional isomers. iupac defines constitutional isomerism as “isomerism between structures differing in constitution and described by different line formulae e.g. ch3och3 and ch3ch2oh.”. recall that there are three types of constitutional isomer commonly seen: chain, positional and functional. I count 11 isomers of "c" 3"h" 6"o". > they are: prop 2 en 1 ol (from commons.wikimedia.org) methoxyethene propanone (from meritnation ) and its tautomer, prop 1 en 2 ol propanal (from commons.wikimedia.org) and its two tautomeric isomers (z) prop 1 en 1 ol and (e) prop 1 en 1 ol then we have cyclopropanol oxetane (r) methyloxirane (s) methyloxirane and that makes 11 isomers!. Key terms. make certain that you can define, and use in context, the key terms below. constitutional. (structural) isomers. stereoisomers. the following flow chart can be used to identify the relationship of two compounds with respect to isomerization: figure 8.1.1 8.1. 1: different types of isomers.

Write All The Acyclic And Cyclic Isomers Of A Compound Having Molecular I count 11 isomers of "c" 3"h" 6"o". > they are: prop 2 en 1 ol (from commons.wikimedia.org) methoxyethene propanone (from meritnation ) and its tautomer, prop 1 en 2 ol propanal (from commons.wikimedia.org) and its two tautomeric isomers (z) prop 1 en 1 ol and (e) prop 1 en 1 ol then we have cyclopropanol oxetane (r) methyloxirane (s) methyloxirane and that makes 11 isomers!. Key terms. make certain that you can define, and use in context, the key terms below. constitutional. (structural) isomers. stereoisomers. the following flow chart can be used to identify the relationship of two compounds with respect to isomerization: figure 8.1.1 8.1. 1: different types of isomers. Functional group isomerism. in this variety of structural isomerism, the isomers contain different functional groups that is, they belong to different families of compounds (different homologous series). example 3: isomers in c 3 h 6 o. a molecular formula c3h6o c 3 h 6 o could be either propanal (an aldehyde) or propanone (a ketone). Answer. step 1: draw the structural formula of the compound. step 2: determine whether it is a stereo or structural isomer. there is no restricted bond rotation around the c c bond, so it is structural isomerism. step 3: determine whether it is a functional group, chain or positional isomerism.

Number Of Structural Isomers Possible In C3h6o Functional group isomerism. in this variety of structural isomerism, the isomers contain different functional groups that is, they belong to different families of compounds (different homologous series). example 3: isomers in c 3 h 6 o. a molecular formula c3h6o c 3 h 6 o could be either propanal (an aldehyde) or propanone (a ketone). Answer. step 1: draw the structural formula of the compound. step 2: determine whether it is a stereo or structural isomer. there is no restricted bond rotation around the c c bond, so it is structural isomerism. step 3: determine whether it is a functional group, chain or positional isomerism.
What Are The Isomers Of C 3 H 6 O

Comments are closed.