New Drug Signals The Beginning Of A Treatment Age For Alzheimers

Drug Discovery Alzheimer S Research Uk July 2, 2024. the food and drug administration on tuesday approved a new drug for alzheimer’s disease, the latest in a novel class of treatments that has been greeted with hope, disappointment. The first new drugs in more than 20 years for the treatment of alzheimer’s disease are likely to be available here soon. ap. in an 18 month trial involving 1795 people aged 50 to 90, the first.

From Mechanisms To Drugs In Alzheimer S Disease The Lancet The food and drug administration has fully approved the first drug shown to slow down alzheimer's disease. the action means that leqembi, whose generic name is lecanemab, should be widely covered. The food and drug administration (fda) recently granted full approval to a new alzheimer’s treatment called lecanemab, which has been shown to moderately slow cognitive and functional decline in early stage cases of the disease. alzheimer’s disease is a progressive disorder that damages and destroys nerve cells in the brain. over time, the. The food and drug administration approved eli lilly’s kisunla on tuesday, july 2, 2024 for mild or early cases of dementia caused by alzheimer’s. (eli lilly and company via ap) washington (ap) — u.s. officials have approved another alzheimer’s drug that can modestly slow the disease, providing a new option for patients in the early. On tuesday, the f.d.a. approved donanemab, a new alzheimer’s medication. we asked experts how the rollout of a similar drug has gone.

Alzheimer S Disease Treatments What To Know About New And Future Drugs The food and drug administration approved eli lilly’s kisunla on tuesday, july 2, 2024 for mild or early cases of dementia caused by alzheimer’s. (eli lilly and company via ap) washington (ap) — u.s. officials have approved another alzheimer’s drug that can modestly slow the disease, providing a new option for patients in the early. On tuesday, the f.d.a. approved donanemab, a new alzheimer’s medication. we asked experts how the rollout of a similar drug has gone. April grant. 202 657 8179. consumer: 888 info fda. fda approved leqembi (lecanemab irmb) for treatment of alzheimer’s disease. leqembi is the second of a new category of medications approved for. Jan. 6, 2023. the food and drug administration on friday approved a new alzheimer’s drug that may modestly slow the pace of cognitive decline early in the disease, but also carries risks of.

Do New Alzheimer S Drugs Signal The End Of The Condition New Scientist April grant. 202 657 8179. consumer: 888 info fda. fda approved leqembi (lecanemab irmb) for treatment of alzheimer’s disease. leqembi is the second of a new category of medications approved for. Jan. 6, 2023. the food and drug administration on friday approved a new alzheimer’s drug that may modestly slow the pace of cognitive decline early in the disease, but also carries risks of.
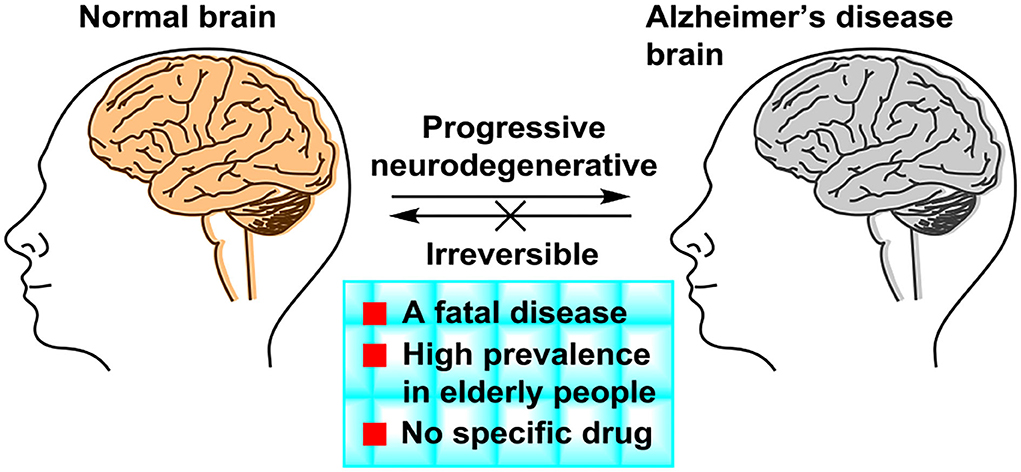
Frontiers Small Molecule Drugs Development For Alzheimer S Disease
Fda Authorizes Aduhelm For Alzheimer S Disease

Comments are closed.