Neurologist Alzheimer S Drug Is Not The Beginning Of The End But The

Neurologist Alzheimer S Drug Is Not The Beginning Of The End But The The development and approval of leqembi, the first fda approved medication proven to slow the progression of alzheimer’s disease, is a milestone. but there is a need to develop drugs with. Dr. lawrence s. honig is a neurologist at newyork presbyterian columbia university irving medical center.he is a professor of neurology at the taub institute for research on alzheimer’s disease and the aging brain, and the gertrude h. sergievsky center, where he directs the new york state funded center of excellence for alzheimer’s disease.
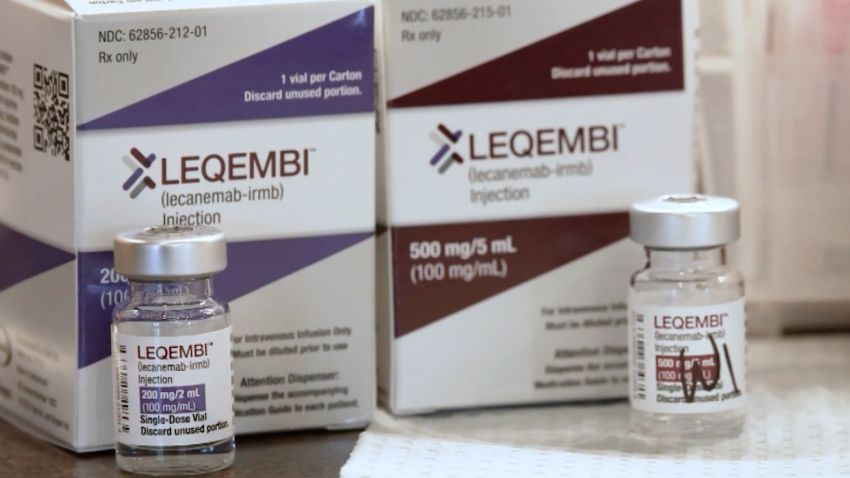
Neurologist Alzheimer S Drug Is Not The Beginning Of The End But The The unusual thing about this drug is that it targets not only the amyloid plaques that are a hallmark of alzheimer’s but also clumps of amyloid, called oligomers, that float around inside and. Last week, a major medical milestone was reached when the food and drug administration approved an alzheimer's medication. the drug, known as leqembi and developed by cambridge based pharmaceutical company biogen, is the first medicine proven to slow the course of the disease in its early stages. July 6, 2023 4:58 pm edt. i t’s a decision that millions of people affected by alzheimer’s disease and their families have been waiting for—the first fully approved drug that treats the. The food and drug administration has fully approved the first drug shown to slow down alzheimer's disease. the action means that leqembi, whose generic name is lecanemab, should be widely covered.

Neurologist Alzheimer S Drug Is Not The Beginning Of The End But The July 6, 2023 4:58 pm edt. i t’s a decision that millions of people affected by alzheimer’s disease and their families have been waiting for—the first fully approved drug that treats the. The food and drug administration has fully approved the first drug shown to slow down alzheimer's disease. the action means that leqembi, whose generic name is lecanemab, should be widely covered. In a large study, the experimental drug donanemab slowed the progression of alzheimer's by about 35%. that's slightly better than the drug leqembi, which was fully approved by the fda on july 6. The alzheimer’s drug leqembi, the first medicine proven to slow the course of the disease, may be able to be given as a set of two weekly shots at home, a study from drugmaker eisai suggests.

Comments are closed.