June 17 2022 Acip Meeting Welcome Coronavirus Disease 2019 Covid 19 Vaccines

June 17 2022 Acip Meeting Welcome Coronavirus Disease 2019 Covid Acip covid 19 vaccines work group : june 17, 2022 june 17, 2022 acip meeting: 2022 jun 17; acip meeting: 2022 jun 17 the centers for disease control and. Acip evidence to recommendations for use of moderna covid 19 vaccine in children ages 6 – 11 years and adolescents ages 12 – 17 years under an emergency use authorization. mmwr, june 28, 2022, vol 71 (26);859–868. interim recommendations of the advisory committee on immunization practices for use of moderna and pfizer biontech covid 19.

Jan 12 2022 Acip Meeting Welcome Cholera Vaccine Covid 19 News Safety and immunogenicity of the pfizer biontech covid 19 vaccine 3 dose primary series in children ages 6 months —4 years acip covid 19 vaccine work group interpretation of safety, immunogenicity and efficacy of moderna & pfizer biontech covid 19 vaccine primary series data. acip meeting review. june 17, 2022. 4. reviewed:. As of june 14, 2022, there were more than 85 million total recorded cases of covid 19 in the united states. 1 additionally, as of june 12, 2022, over 5.1 million cases occurred among children ages 5 – 11 years and more than 5.6 million cases occurred among adolescents ages 12 – 17 years. 2 furthermore, in relation to the rates of covid 19 cases by vaccination status and age group, as of. On june 17, 2022, the food and drug administration (fda) issued emergency use authorization (eua) amendments for the mrna 1273 (moderna) covid 19 vaccine for use in children aged 6 months 5 years, administered as 2 doses (25 µg [0.25 ml] each), 4 weeks apart, and bnt162b2 (pfizer biontech) covid 19 …. Covid 19 (coronavirus disease 2019) is a disease caused by the sars cov 2 virus. covid 19 most often causes respiratory symptoms that can feel much like a cold, the flu, or pneumonia. most people with covid 19 have mild symptoms, but some people become severely ill. you can protect against severe covid 19 disease with vaccination.
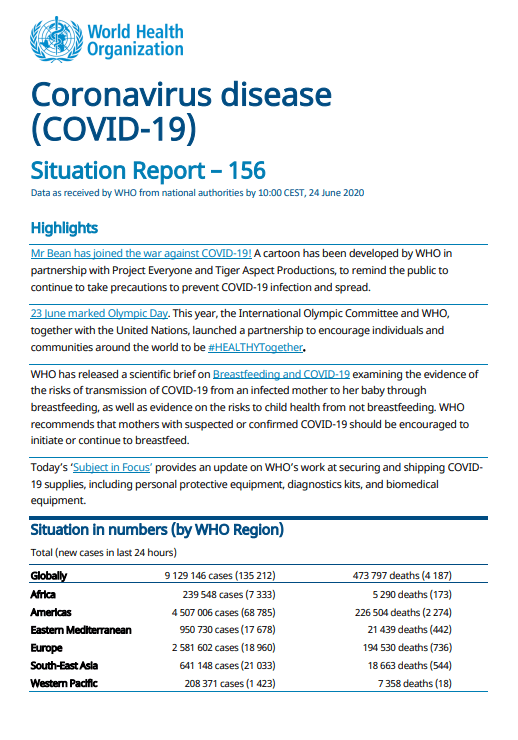
Coronavirus Disease 2019 Covid 19 Situation Report 156 Cde On june 17, 2022, the food and drug administration (fda) issued emergency use authorization (eua) amendments for the mrna 1273 (moderna) covid 19 vaccine for use in children aged 6 months 5 years, administered as 2 doses (25 µg [0.25 ml] each), 4 weeks apart, and bnt162b2 (pfizer biontech) covid 19 …. Covid 19 (coronavirus disease 2019) is a disease caused by the sars cov 2 virus. covid 19 most often causes respiratory symptoms that can feel much like a cold, the flu, or pneumonia. most people with covid 19 have mild symptoms, but some people become severely ill. you can protect against severe covid 19 disease with vaccination. 08 23 2021. fda approves first covid 19 vaccine. today, the u.s. food and drug administration approved the first covid 19 vaccine, known as the pfizer biontech covid 19 vaccine, now marketed as. Introduction; epidemiology of covid 19 in young children; updates on vaccine effectiveness of covid 19 vaccines in children and adolescents.

Coronavirus Disease 2019 Covid 19 Situation Report 132 Cde 08 23 2021. fda approves first covid 19 vaccine. today, the u.s. food and drug administration approved the first covid 19 vaccine, known as the pfizer biontech covid 19 vaccine, now marketed as. Introduction; epidemiology of covid 19 in young children; updates on vaccine effectiveness of covid 19 vaccines in children and adolescents.
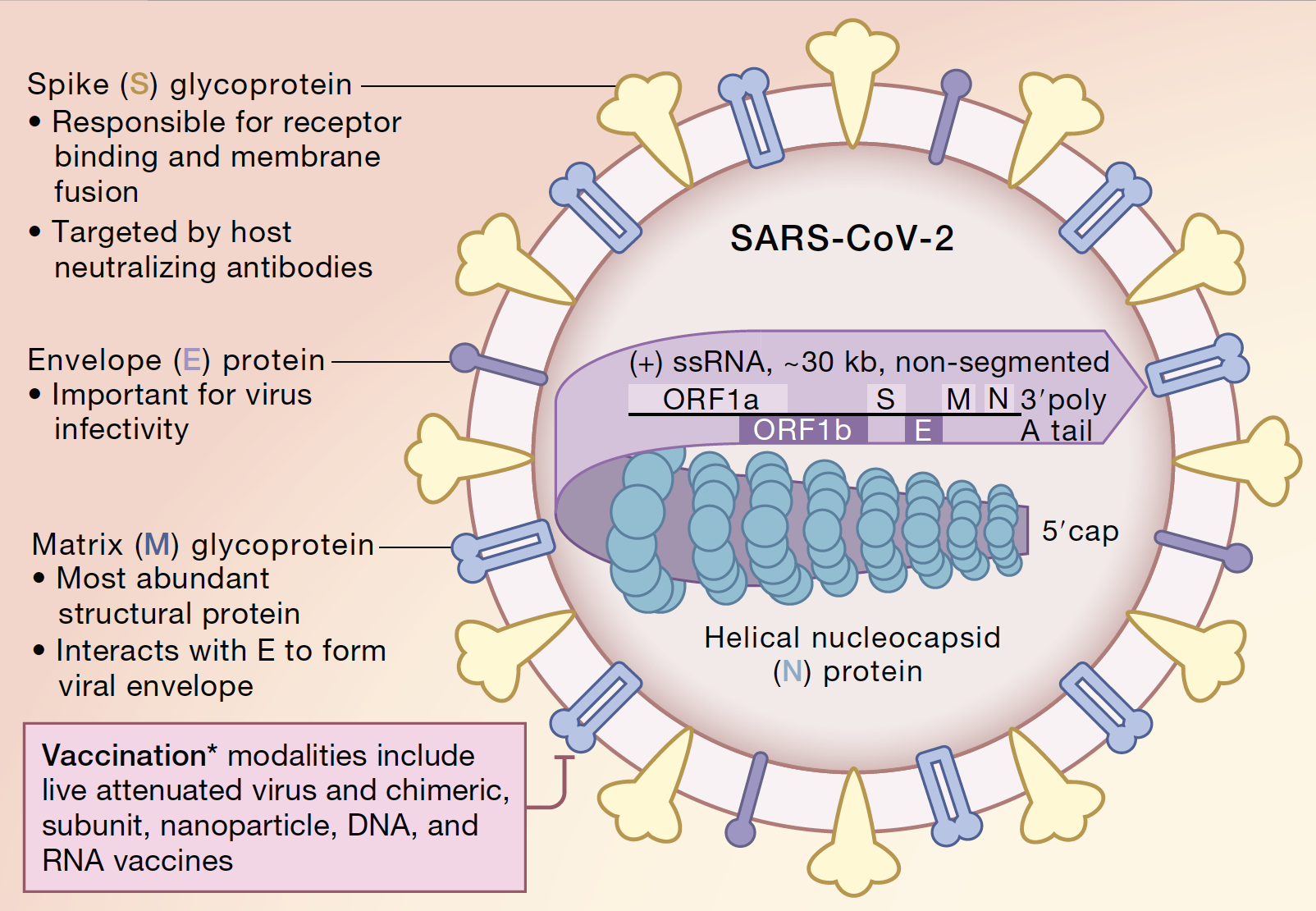
Research Related To Covid 19 Endowment For Basic Sciences Michigan

June 17 2022 Acip Meeting Bnt162b2 Mrna Covid 19 Vaccines In Young

Comments are closed.