Internal Energy Work And Heat Heat And Thermodynamics Physics
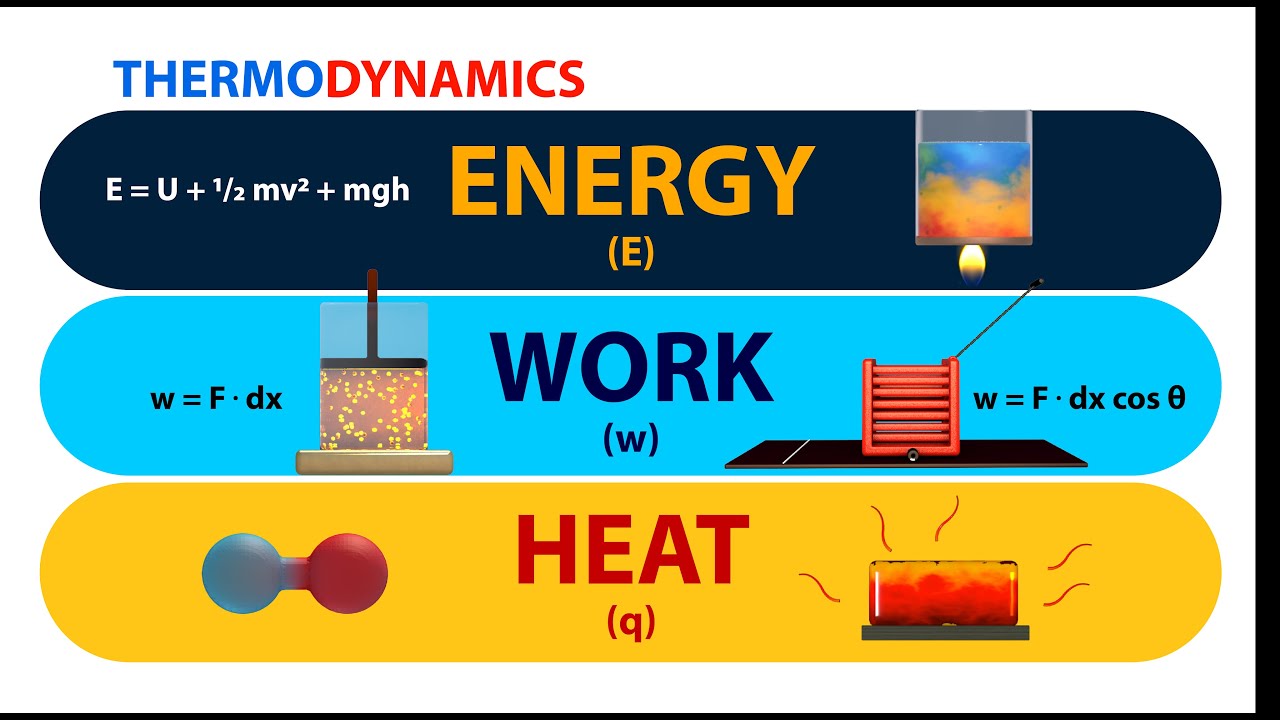
Thermodynamics Energy Work And Heat Animation Youtube This page titled 3.3: work, heat, and internal energy is shared under a cc by 4.0 license and was authored, remixed, and or curated by openstax via source content that was edited to the style and standards of the libretexts platform. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. Energy is transferred along with the genetic material and so obeys the first law of thermodynamics. energy is transferred—not created or destroyed—in the process. when work is done on a cell or heat transfers energy to a cell, the cell’s internal energy increases. when a cell does work or loses heat, its internal energy decreases.

Ppt L 20 Thermodynamics 5 Powerpoint Presentation Id 4294508 The internal energy of a system can change if heat is added or removed, or if work is done on or by the system. the formula that represents this is: Δu = q– w. here, (∆u) is the change in internal energy, (q) is the heat added to the system, and (w) is the work done by the system. relation between enthalpy and internal energy. The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring energy into and out of the system. the first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done. The first law of thermodynamics ties together work and heat with the concept of internal energy (u). it can be succinctly stated as: \delta u = q – w Δu=q−w. where \delta u Δu is the change in internal energy of the system, q q is the heat added to the system, and w w is the work done by the system. this equation signifies that the change. Summary. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. heat is the energy transferred between two objects (or two parts of a system) because of a temperature difference. internal energy of a thermodynamic system is its total mechanical energy.

First Law Of Thermodynamics Basic Introduction Internal Energy Heat The first law of thermodynamics ties together work and heat with the concept of internal energy (u). it can be succinctly stated as: \delta u = q – w Δu=q−w. where \delta u Δu is the change in internal energy of the system, q q is the heat added to the system, and w w is the work done by the system. this equation signifies that the change. Summary. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. heat is the energy transferred between two objects (or two parts of a system) because of a temperature difference. internal energy of a thermodynamic system is its total mechanical energy. The first law of thermodynamics relates the internal energy change, work done by the system, and the heat transferred to the system in a simple equation. the internal energy is a function of state and is therefore fixed at any given point regardless of how the system reaches the state. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. in equation form, the first law of thermodynamics is. Δu = q − w. (15.1.1) (15.1.1) Δ u = q − w. here Δu Δ u is the change in internal energy u u of the system.
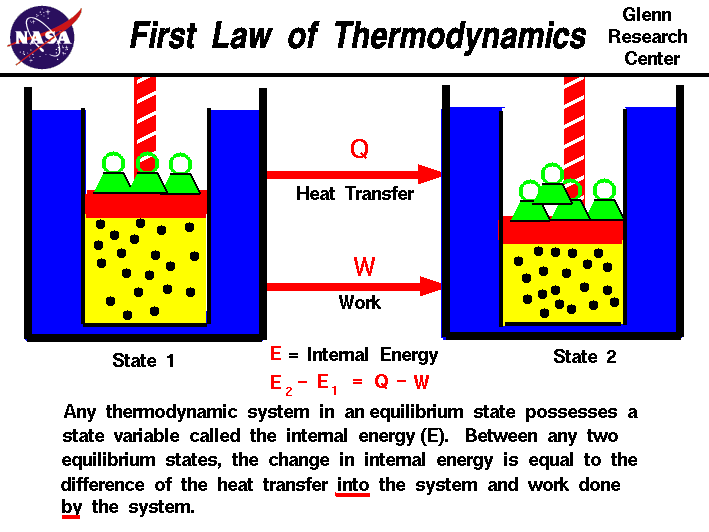
First Law Of Thermodynamics The first law of thermodynamics relates the internal energy change, work done by the system, and the heat transferred to the system in a simple equation. the internal energy is a function of state and is therefore fixed at any given point regardless of how the system reaches the state. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. in equation form, the first law of thermodynamics is. Δu = q − w. (15.1.1) (15.1.1) Δ u = q − w. here Δu Δ u is the change in internal energy u u of the system.

Comments are closed.