Internal Energy Enthalpy And Specific Heats Of Ideal Gas

Ideal Gases Specific Heat Internal Energy Enthalpy Thermodynamics Learn about how specific heat, internal energy and enthalpy work with ideal gases. we go through constant volume and constant pressure specific volume, how e. An ideal gas with specific heats independent of temperature, and , is referred to as a perfect gas. for example, monatomic gases and diatomic gases at ordinary temperatures are considered perfect gases. to make this distinction the terminology "a perfect gas with constant specific heats" is used throughout the notes. in some textbooks perfect.

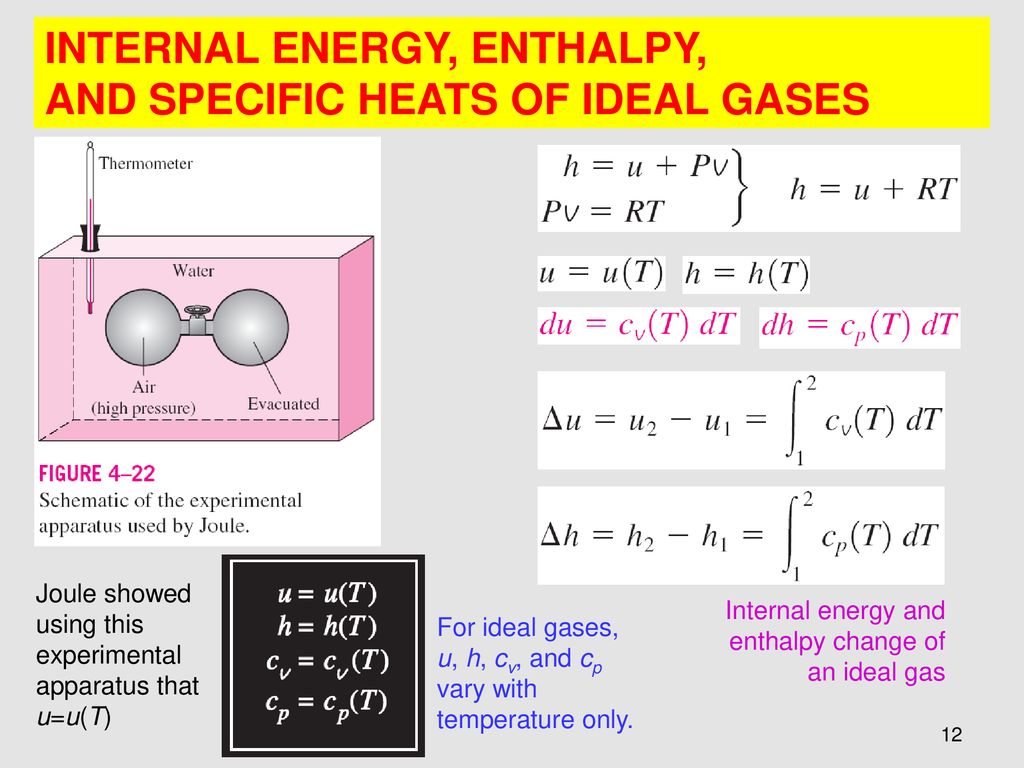
Example Using Specific Heat To Calculate Ideal Gas Internal Energy The monoatomic ideal gas constant volume specific heat c v ¯ is one of the more remarkable theoretical results the first four periodic gases in the periodic table all have molar specific heats of 12.5 j mol 1 k 1 under conditions of constant volume, and deviations for the larger ideal gases are minor and only in the third significant figure. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. [1] the ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. the requirement of zero interaction can often be. Enthalpy ideal gas. enthalpy is a thermodynamic property that represents the systems internal energy plus the product of the systems pressure and volume. in other words, it represents the total heat content of a system. for an ideal gas, the definition of enthalpy and the equation of state is as follows. (eq 2) h = u r t = {h = u p ν p ν. Average particle speed in a gas. we can use equation 5.5.2 and equation 5.5.14 to draw another conclusion about the particles in this gas: u = 3 2nkbt ⇒ u ≡ u n = 3 2kbt. this tells us that the average energy per particle u is a constant times the temperature of the gas.

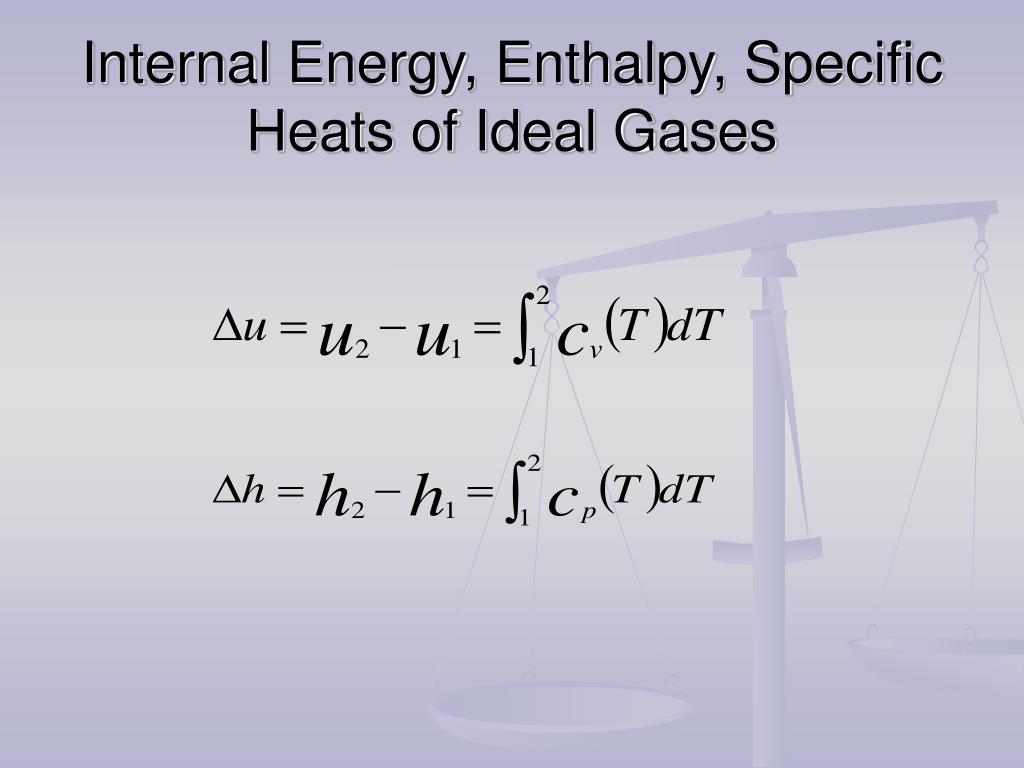
Ppt Calculating Internal Energy And Enthalpy Changes Powerpoint Enthalpy ideal gas. enthalpy is a thermodynamic property that represents the systems internal energy plus the product of the systems pressure and volume. in other words, it represents the total heat content of a system. for an ideal gas, the definition of enthalpy and the equation of state is as follows. (eq 2) h = u r t = {h = u p ν p ν. Average particle speed in a gas. we can use equation 5.5.2 and equation 5.5.14 to draw another conclusion about the particles in this gas: u = 3 2nkbt ⇒ u ≡ u n = 3 2kbt. this tells us that the average energy per particle u is a constant times the temperature of the gas. The internal energy of an ideal gas is proportional to its amount of substance (number of moles) and to its temperature. where is the isochoric (at constant volume) molar heat capacity of the gas; is constant for an ideal gas. the internal energy of any gas (ideal or not) may be written as a function of the three extensive properties. The specific heats of gases are generally expressed as molar specific heats. for a monoatomic ideal gas the internal energy is all in the form of kinetic energy, and kinetic theory provides the expression for that energy, related to the kinetic temperature. the expression for the internal energy is . two specific heats are defined for gases.

Lecture 13 Ideal Gases Internal Energy Enthalpy And Specific Heats The internal energy of an ideal gas is proportional to its amount of substance (number of moles) and to its temperature. where is the isochoric (at constant volume) molar heat capacity of the gas; is constant for an ideal gas. the internal energy of any gas (ideal or not) may be written as a function of the three extensive properties. The specific heats of gases are generally expressed as molar specific heats. for a monoatomic ideal gas the internal energy is all in the form of kinetic energy, and kinetic theory provides the expression for that energy, related to the kinetic temperature. the expression for the internal energy is . two specific heats are defined for gases.
Chapter 4 Energy Analysis Of Closed Systems Ppt Download
Ppt Properties Of Pure Substances Powerpoint Presentation Free

Comments are closed.