Intermolecular Interparticle Forces London Dispersion Forces Ap Chem Unit 3 Topic 1a

Intermolecular Interparticle Forces London Dispersion Forces Ap In this video, mr. krug gives an introduction to intermolecular and interparticle forces and discusses london dispersion forces. A measure of an atom's ability to attract electrons. properties of weak intermolecular forces: high vp, high volatility (likelihood of turning into a gas), likely to be found in a gaseous state. properties of strong intermolecular forces: high bp, mp, likely to be found as a solid, high viscocity. viscosity.
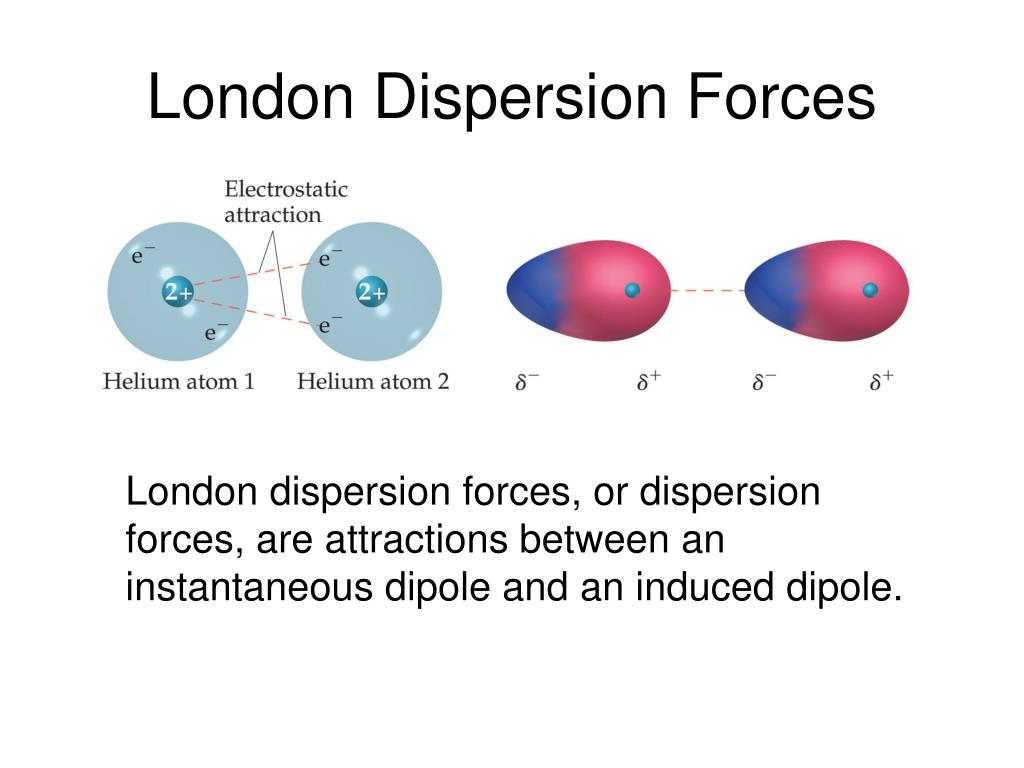
Ppt Intermolecular Forces Powerpoint Presentation Free Download Id Study with quizlet and memorize flashcards containing terms like forces are a result of coulombic interactions between temporary and fluctuating dipoles. these forces are often the strongest net intermolecular force between large molecules, london dispersion forces: an increase in contact area between molecules, along with an increase in polarizability of the molecules. London dispersion forces. london dispersion forces are a weak type of intermolecular forces produced by small instantaneous dipoles that occur in nonpolar molecules. instantaneous dipoles are the result of an electron's motion in one atom affecting another atom. the average distribution of electrons in a nonpolar molecule is symmetrical. Mole fraction. the moles of a substance in a mixture divided by the total moles in a mixture. you can find partial pressure if . you multiply total pressure by mole fraction. study with quizlet and memorize flashcards containing terms like intermolecular forces, intramolecular forces, london dispersion forces and more. Learning objectives. for the ap chemistry exam, you should learn to identify and explain the different types of intermolecular forces (london dispersion forces, dipole dipole interactions, hydrogen bonding, and ion dipole interactions), understand the factors that affect the strength of these forces, and describe how intermolecular forces influence physical properties such as boiling and.

London Dispersion Forces Temporary Dipole Induced Dipole Mole fraction. the moles of a substance in a mixture divided by the total moles in a mixture. you can find partial pressure if . you multiply total pressure by mole fraction. study with quizlet and memorize flashcards containing terms like intermolecular forces, intramolecular forces, london dispersion forces and more. Learning objectives. for the ap chemistry exam, you should learn to identify and explain the different types of intermolecular forces (london dispersion forces, dipole dipole interactions, hydrogen bonding, and ion dipole interactions), understand the factors that affect the strength of these forces, and describe how intermolecular forces influence physical properties such as boiling and. Unit 3 intermolecular forces and properties. key topics: intermolecular forces, states of matter, boiling point, melting point, spectroscopy, ideal gas law, solutions. 📄️ intermolecular forces. ap chem guide's crash course on intermolecular forces. 📄️ properties of solids. ap chem guide's crash course on properties of solids. Justify your answer with a calculation. the balanced equation for the combustion of 2.00 mol of propene is. 2 c 2 h (g) 9 o 2 (g) → 6 c o 2 (g) 6 h 2 o (g). ️answer explanation. (a) the pvc beads sink. the spacing between chains is similar, but a cl atom has a greater mass than. both substances have dipole dipole interactions and london.

London Dispersion Forces Intermolecular Forces And Properties Ap Unit 3 intermolecular forces and properties. key topics: intermolecular forces, states of matter, boiling point, melting point, spectroscopy, ideal gas law, solutions. 📄️ intermolecular forces. ap chem guide's crash course on intermolecular forces. 📄️ properties of solids. ap chem guide's crash course on properties of solids. Justify your answer with a calculation. the balanced equation for the combustion of 2.00 mol of propene is. 2 c 2 h (g) 9 o 2 (g) → 6 c o 2 (g) 6 h 2 o (g). ️answer explanation. (a) the pvc beads sink. the spacing between chains is similar, but a cl atom has a greater mass than. both substances have dipole dipole interactions and london.

Ppt Intermolecular Forces Powerpoint Presentation Free Download Id

Comments are closed.