Interactive Example 7 2 The Energy Of A Photon

Solved Interactive Example The Energy Of A Photon When A Chegg Playlist: playlist?list=plgzjchhyqf9ndg2feyn8 sscobe2vspcc&si=9qojsl29nqkjp5azplaylist: playlist?list=plgzjchhyqf. Note: this is the energy of one photon and can also be written as 4.30 x 10 19 j photon. calculating the energy based on the moles of photons. example: what is the energy of 1 mol of photons, in kj, for a 724 nm red light? solution: remember, the formula correlating the energy and the wavelength is for one photon. therefore, we can calculate.
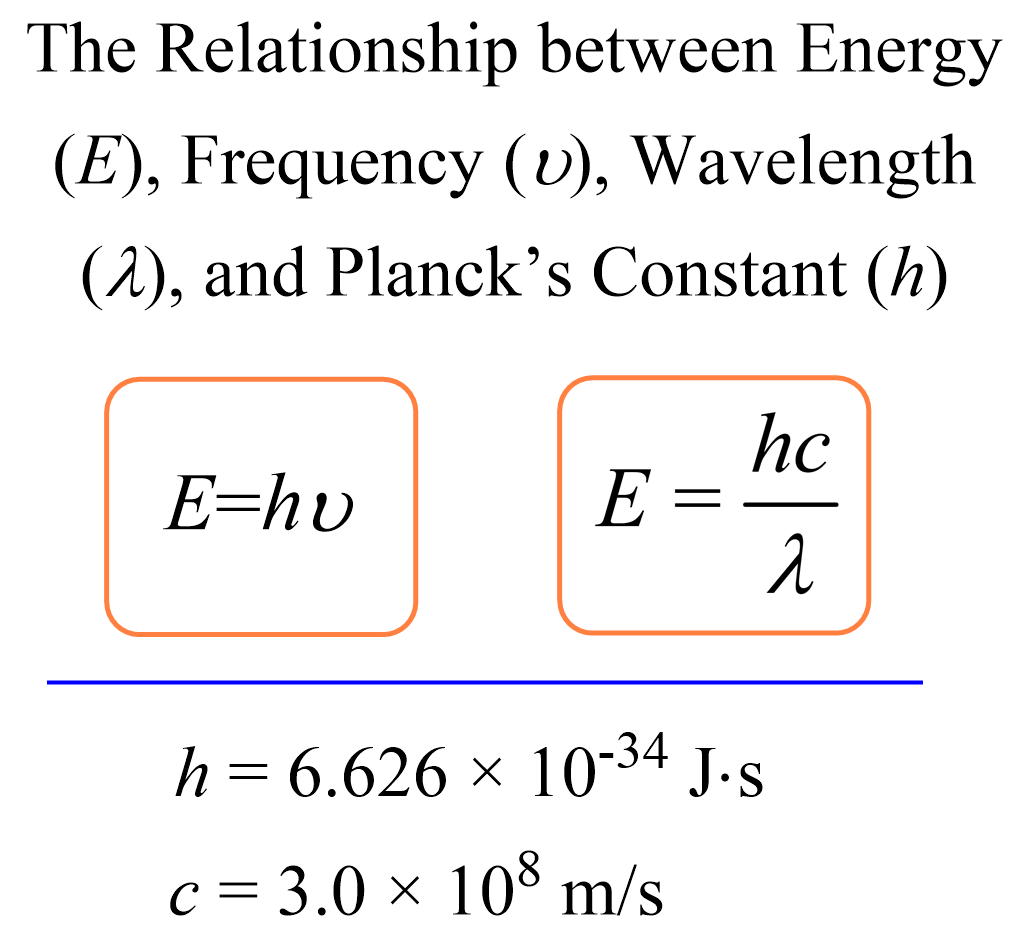
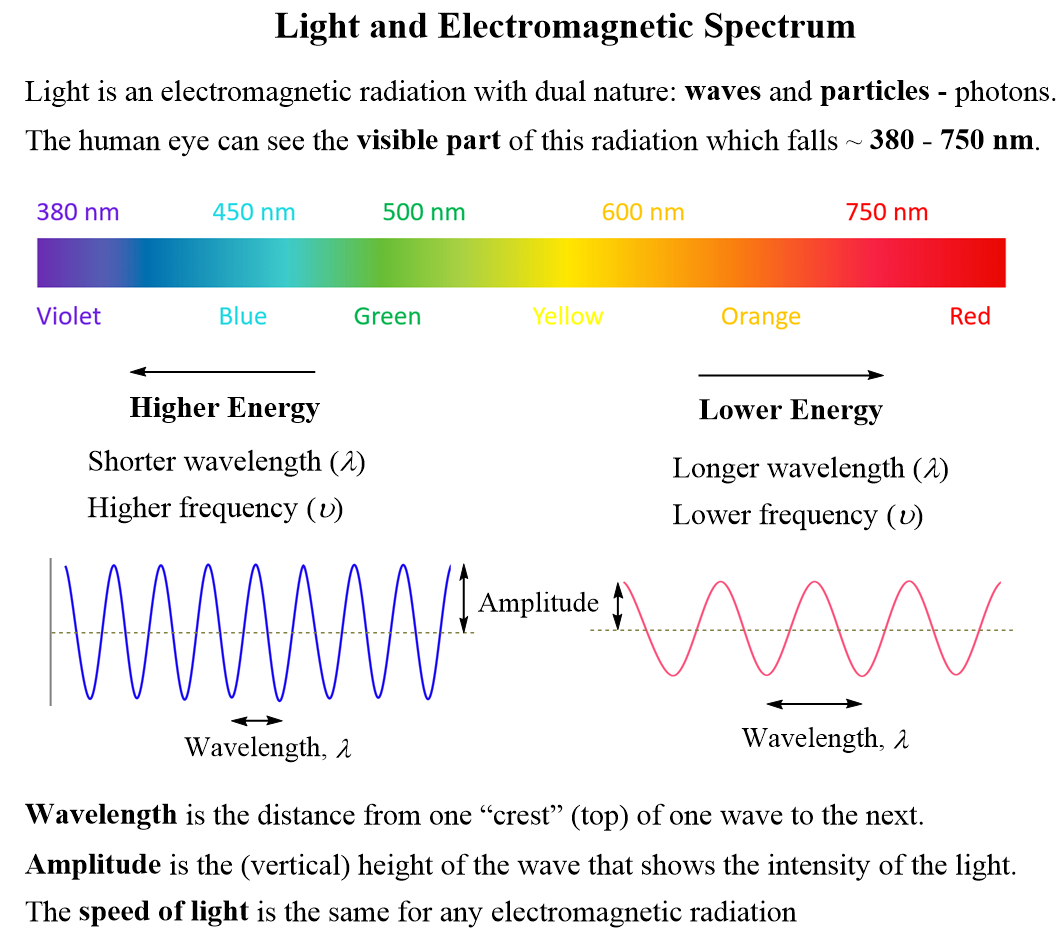
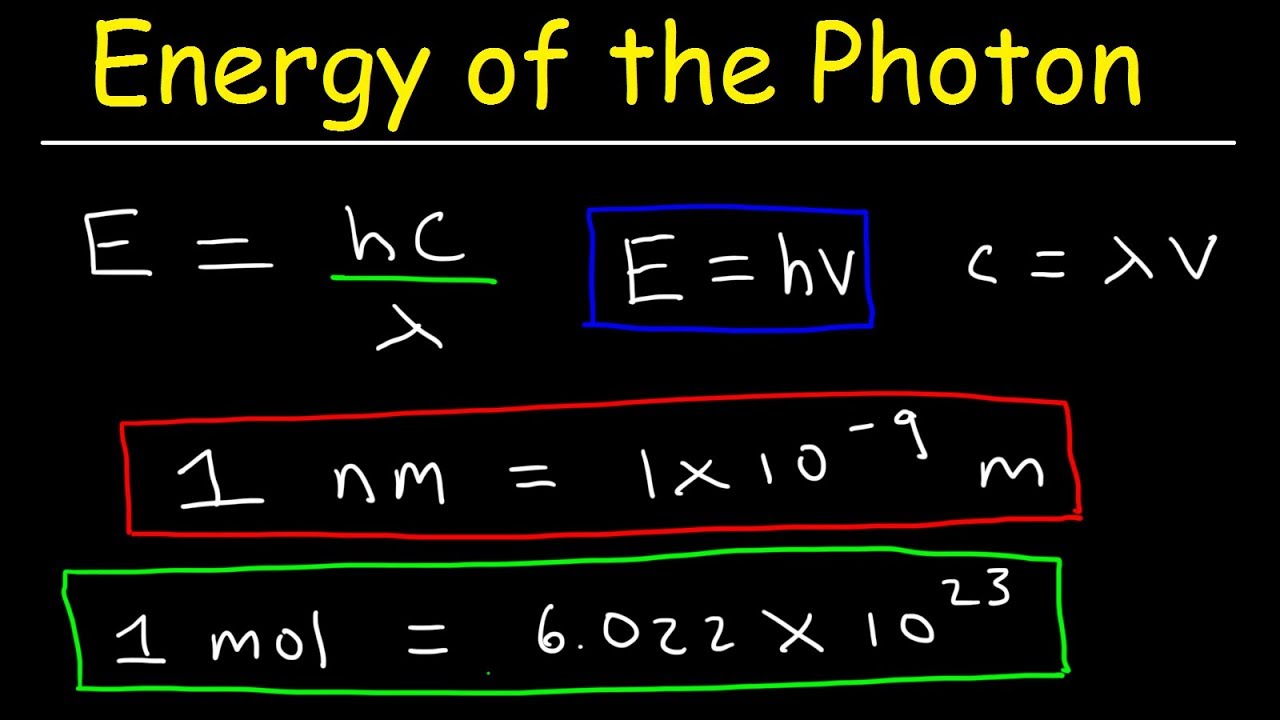
Calculating The Energy Of A Photon Chemistry Steps Solution. divide the given energy of the photon by the planck constant to find the frequency of the green colored light. interactive example the energy of a photon when a green colored light is observed, the increment of energy (the quantum) that is emitted is 4.04x10 19 j. what is the wavelength (in nm) of this light?. The energy of a single photon is a small number because the planck constant is ridiculously tiny. the energy of a single photon of green light of a wavelength of 520 nm has an energy of 2.38 ev. you can use the photon energy calculator to explore further the relationship between the photon energy and its frequency or wavelength. A photon is a quantum of em radiation. its energy is given by e = hf and is related to the frequency f and wavelength λ of the radiation by. e = hf = hc λ (energy of a photon) e = h f = h c λ (energy of a photon), where e is the energy of a single photon and c is the speed of light. when working with small systems, energy in ev is often useful. Table 29.3.1 29.3. 1. gamma rays, a form of nuclear and cosmic em radiation, can have the highest frequencies and, hence, the highest photon energies in the em spectrum. for example, a γ γ ray photon with f = 1021 hz f = 10 21 h z has an energy e = hf = 6.63 ×10−13 j = 4.14mev e = h f = 6.63 × 10 − 13 j = 4.14 m e v.

Ppt Light Photon Energies And Atomic Spectra Powerpoint A photon is a quantum of em radiation. its energy is given by e = hf and is related to the frequency f and wavelength λ of the radiation by. e = hf = hc λ (energy of a photon) e = h f = h c λ (energy of a photon), where e is the energy of a single photon and c is the speed of light. when working with small systems, energy in ev is often useful. Table 29.3.1 29.3. 1. gamma rays, a form of nuclear and cosmic em radiation, can have the highest frequencies and, hence, the highest photon energies in the em spectrum. for example, a γ γ ray photon with f = 1021 hz f = 10 21 h z has an energy e = hf = 6.63 ×10−13 j = 4.14mev e = h f = 6.63 × 10 − 13 j = 4.14 m e v. Calculate the photon energy in ev for 100 nm vacuum uv, and estimate the number of molecules it could ionize or break apart. strategy. using the equation e = hf e = hf and appropriate constants, we can find the photon energy and compare it with energy information in table 29.1. solution. the energy of a photon is given by. The frequency of a photon is 200 h z. determine the energy of the photon. 5. figure out the energy of a photon that has a frequency equal to 5000 h z. 6. figure out the energy of a photon that has.
Calculating The Energy Of A Photon Chemistry Steps Calculate the photon energy in ev for 100 nm vacuum uv, and estimate the number of molecules it could ionize or break apart. strategy. using the equation e = hf e = hf and appropriate constants, we can find the photon energy and compare it with energy information in table 29.1. solution. the energy of a photon is given by. The frequency of a photon is 200 h z. determine the energy of the photon. 5. figure out the energy of a photon that has a frequency equal to 5000 h z. 6. figure out the energy of a photon that has.

How To Calculate The Energy Of A Photon Physics Study
How To Calculate The Energy Of A Photon Given Frequency Wavelength In

Comments are closed.