How To Draw Structural Isomers Containing Oxygen C4h10o

How To Draw Structural Isomers Containing Oxygen C4h10o Youtube Step 1. draw the possible molecules that exist if the formula didn't have oxygen in it. for c4h10o, this includes both isomers of c4h10: butane and 2 methylp. Kekulé formula. a structural formula displays the atoms of the molecule in the order they are bonded. it also depicts how the atoms are bonded to one another, for example single, double, and triple covalent bond. covalent bonds are shown using lines. the number of dashes indicate whether the bond is a single, double, or triple covalent bond.
All Isomers Of C4h10o Learn how to draw all the isomers of a given molecular formula. Kekulé formula. a structural formula displays the atoms of the molecule in the order they are bonded. it also depicts how the atoms are bonded to one another, for example single, double, and triple covalent bond. covalent bonds are shown using lines. the number of dashes indicate whether the bond is a single, double, or triple covalent bond. See below. > we know from the formula that these isomers contain no rings or double bonds. the isomers must be alcohols and ethers. let's start by drawing the alcohols. start with four carbons in a row and put an "oh" group in every possible position. this gives us 1. "ch" 3"ch" 2"ch" 2"ch" 2"oh", butan 1 ol and 2. Functional group isomerism. in this variety of structural isomerism, the isomers contain different functional groups that is, they belong to different families of compounds (different homologous series). example 3: isomers in c 3 h 6 o. a molecular formula c3h6o c 3 h 6 o could be either propanal (an aldehyde) or propanone (a ketone).
All Isomers Of C4h10o See below. > we know from the formula that these isomers contain no rings or double bonds. the isomers must be alcohols and ethers. let's start by drawing the alcohols. start with four carbons in a row and put an "oh" group in every possible position. this gives us 1. "ch" 3"ch" 2"ch" 2"ch" 2"oh", butan 1 ol and 2. Functional group isomerism. in this variety of structural isomerism, the isomers contain different functional groups that is, they belong to different families of compounds (different homologous series). example 3: isomers in c 3 h 6 o. a molecular formula c3h6o c 3 h 6 o could be either propanal (an aldehyde) or propanone (a ketone). The importance of drawing geometric isomers properly. it’s very easy to miss geometric isomers in exams if you take short cuts in drawing the structural formulae. for example, it is very tempting to draw but 2 ene as. ch 3 ch=chch 3. if you write it like this, you will almost certainly miss the fact that there are geometric isomers. There are 7 constitutional structural isomers of c 4 h 10 o, but 8 isomers of c 4 h 10 o including the r s isomers. (1) butan 1 ol, , , is a primary alcohol (2) butan 2 ol, , is a secondary alcohol. this has a chiral (asymmetric) carbon atom (2nd c), so can also exhibit r s stereoisomerism.
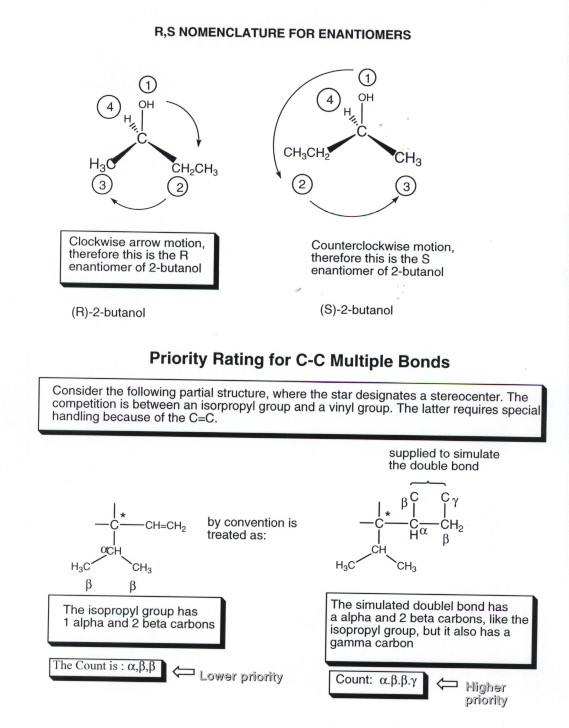
Isomers Of C4h10o The importance of drawing geometric isomers properly. it’s very easy to miss geometric isomers in exams if you take short cuts in drawing the structural formulae. for example, it is very tempting to draw but 2 ene as. ch 3 ch=chch 3. if you write it like this, you will almost certainly miss the fact that there are geometric isomers. There are 7 constitutional structural isomers of c 4 h 10 o, but 8 isomers of c 4 h 10 o including the r s isomers. (1) butan 1 ol, , , is a primary alcohol (2) butan 2 ol, , is a secondary alcohol. this has a chiral (asymmetric) carbon atom (2nd c), so can also exhibit r s stereoisomerism.

Structural Isomers Of C4h10o

All Isomers Of C4h10o

Comments are closed.