How To Calculate The Percent Yield And Theoretical Yield Youtube

How To Calculate The Percent Yield And Theoretical Yield Youtube This chemistry video tutorial explains how to calculate the theoretical yield and percent yield.introduction to moles: www . This video shows you how to calculate the theoretical and percent yield in chemistry. the theoretical yield is the maximum amount of product that can be pro.

How To Calculate Theoretical Yield And Percent Yield Youtube This chemistry video tutorial explains how to calculate the percent yield, actual yield and theoretical yield of a product produced in a chemical reaction gi. The percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage: \[\text{percent yield} = \frac{\text{actual yield}}{\text{theoretical yield}} \times 100\%\nonumber \] percent yield is very important in the manufacture of products. much time and money is spent improving the percent yield for chemical. The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100 to give a percentage: percent yield = actual yield (g) theoretical yield(g) × 100%. the method used to calculate the percent yield of a reaction is illustrated in example 4.3.4. Percent yield formula and definition. percent yield is the actual yield divided by the theoretical yield multiplied by 100%. in chemistry, percent yield is a comparison of actual yield to theoretical yield, expressed as a percentage. here is a look at the percent yield formula, how to calculate it, and why it may be less than or greater than 100%.
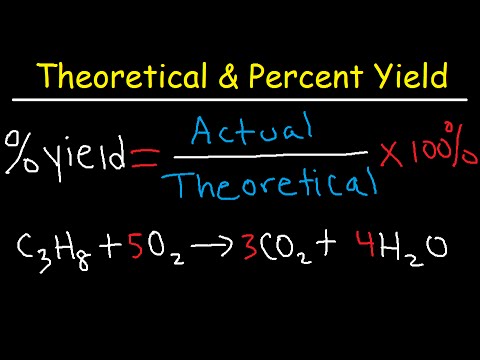
How To Calculate Theoretical Yield And Percent Yield Youtube The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100 to give a percentage: percent yield = actual yield (g) theoretical yield(g) × 100%. the method used to calculate the percent yield of a reaction is illustrated in example 4.3.4. Percent yield formula and definition. percent yield is the actual yield divided by the theoretical yield multiplied by 100%. in chemistry, percent yield is a comparison of actual yield to theoretical yield, expressed as a percentage. here is a look at the percent yield formula, how to calculate it, and why it may be less than or greater than 100%. Problem 1. you mix of mgcl 2 and of agno 3 and recover of agcl. calculate the yield. mgcl 2 and agno 3 react according to the following equation: problem 2. you drop of elemental sodium (na) into of water and collect of h 2. calculate the percent yield. sodium and water react according to the following reaction:. Now, the theoretical yield formula may seem challenging to understand, so we will show you a quick guide on how to calculate the theoretical yield. the measurements you need are the mass of the reagents, their molecular weights, the stoichiometry of the reaction (found from the balanced equation), and the molecular weight of the desired product.

Theoretical And Percent Yield Youtube Problem 1. you mix of mgcl 2 and of agno 3 and recover of agcl. calculate the yield. mgcl 2 and agno 3 react according to the following equation: problem 2. you drop of elemental sodium (na) into of water and collect of h 2. calculate the percent yield. sodium and water react according to the following reaction:. Now, the theoretical yield formula may seem challenging to understand, so we will show you a quick guide on how to calculate the theoretical yield. the measurements you need are the mass of the reagents, their molecular weights, the stoichiometry of the reaction (found from the balanced equation), and the molecular weight of the desired product.

How To Calculate Theoretical Yield And Percent Yield Topics In

R2 1 4 Theoretical Yield And Percent Yield Youtube

Comments are closed.