Heat Internal Energy And Work Thermodynamics Class 11th Youtube

Heat Internal Energy And Work Thermodynamics Class 11th Youtube Heat, internal energy and work donewatch more videos at tutorialspoint videotutorials index ecture by: mr. pradeep kshetrapal, tutorials p. This video includes a detailed explanation of 1) thermodynamics2) thermal equilibrium3) zeroth law of thermodynamics4) internal energy, heat & workclass 11 p.

Heat Internal Energy Work Done Lect No 2 Class 11th 🔰click here to enroll in nexus english batch for free & get access to class notes & other things: physicswallah.onelink.me zazb widie96h 📲 pw app w. Thermodynamics class 11 notes physics chapter 12. • the branch of physics which deals with the study of transformation of heat into other forms of energy and vice versa is called thermodynamics. thermodynamics is a macroscopic science. it deals with bulk systems and does not go into the. molecular constitution of matter. such that it has a. It is denoted by the letter u, measured in joules (j). it depends on the state of the molecules and their random movements. we can increase the internal energy by increasing the supply of heat. the formula for internal energy is given as, Δu = q w, where Δu = internal energy of the system, q = heat supplied to the system, and w = work done. Learn the concepts of class 11 physics thermodynamics with videos and stories. explain heat as an internal energy of matter and give the unit of heat as calorie. heat flows from hot body to cold body. define internal energy, and state that it is a state variable. state on what factors the internal energy depends, and describe a few ways to change internal energy of a system. difference between.
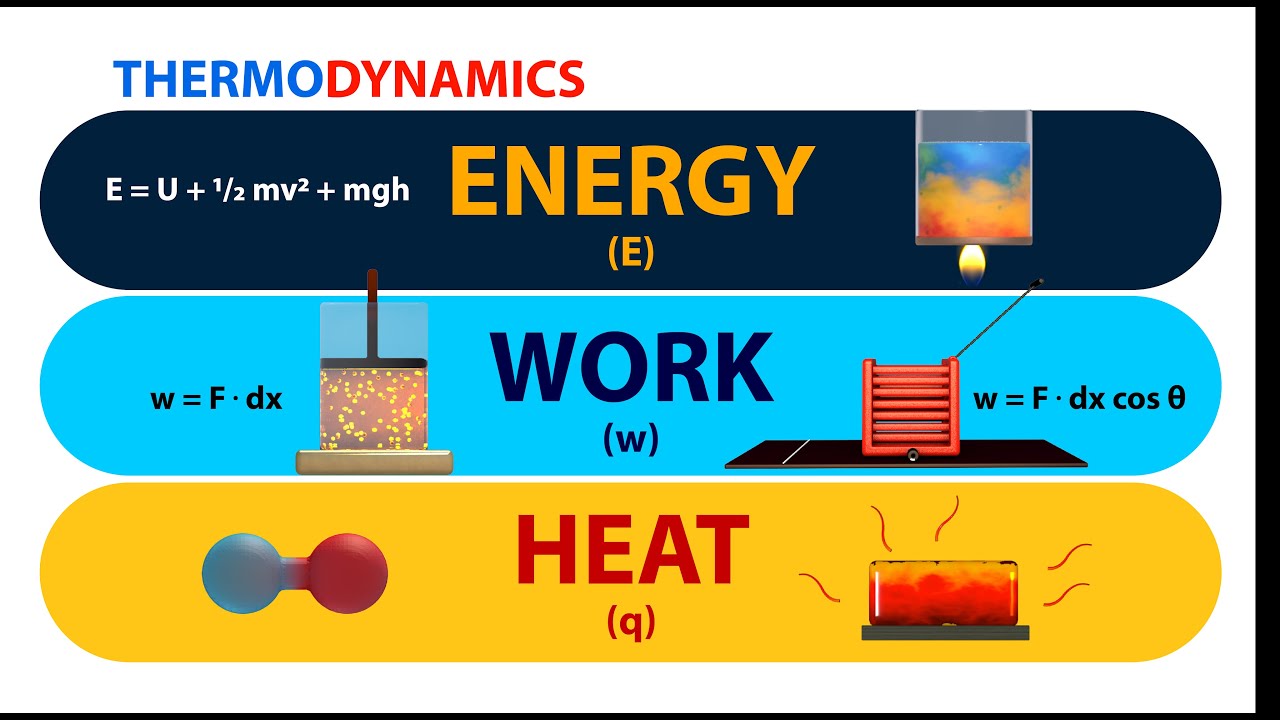
Thermodynamics Energy Work And Heat Animation Youtube It is denoted by the letter u, measured in joules (j). it depends on the state of the molecules and their random movements. we can increase the internal energy by increasing the supply of heat. the formula for internal energy is given as, Δu = q w, where Δu = internal energy of the system, q = heat supplied to the system, and w = work done. Learn the concepts of class 11 physics thermodynamics with videos and stories. explain heat as an internal energy of matter and give the unit of heat as calorie. heat flows from hot body to cold body. define internal energy, and state that it is a state variable. state on what factors the internal energy depends, and describe a few ways to change internal energy of a system. difference between. U = u (t) and enthalpy is given as: h = u pv. using the ideal gas equation, pv = rt. h = u rt. in the above equation, r is the universal gas constant. enthalpy of an ideal gas is given as: h = h (t) we know that specific heats at constant pressure and volume are temperature dependent and can be given as:. The work done by the system in the cycle is. learn the concepts of class 11 physics thermodynamics with videos and stories. explain heat as an internal energy of matter and give the unit of heat as calorie. heat flows from hot body to cold body. define internal energy, and state that it is a state variable.

Thermodynamics Heat Work Internal Energy L3 Class 11 Chemistry U = u (t) and enthalpy is given as: h = u pv. using the ideal gas equation, pv = rt. h = u rt. in the above equation, r is the universal gas constant. enthalpy of an ideal gas is given as: h = h (t) we know that specific heats at constant pressure and volume are temperature dependent and can be given as:. The work done by the system in the cycle is. learn the concepts of class 11 physics thermodynamics with videos and stories. explain heat as an internal energy of matter and give the unit of heat as calorie. heat flows from hot body to cold body. define internal energy, and state that it is a state variable.

Comments are closed.