General Chemistry I Chem 1411 Ch 2 Atoms Molecules And Ions Part 2

General Chemistry I Chem 1411 Ch 2 Atoms Molecules And Ions Part 2 0:00 section 2.4 atomic weights: calculate the average atomic mass of an element from the isotopic masses and isotopic abundances.18:24 section 2.5 the perio. Theory that atoms are fundamental building blocks of matter. 1. each element is composed of extremely small particles called atoms. 2. all atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are diff. from the atoms of all other elements. total mass of substances present at the end of.

Chem 1411 Chapter 2 Atoms Molecules And Ions 2 Chem 1411 Studocu Chem 1411 chapter 3 notes part 1: chemical equations, reaction types and predictability, chapter 3 notes part 2 formula weight, molecular weight, moles, percent composition. chapter 3 notes part 3 theoretical yield, percent yield, combustion analysis, and limiting reactants. Study with quizlet and memorize flashcards containing terms like consider the following selected postulates of dalton's atomic theory:(i) each element is composed of extremely small particles called atoms. (ii) atoms are indivisible. (iii) atoms of a given element are identical. (iv) atoms of different elements are different and have different properties. which of the postulates is(are) no. Each element is composed of extremely small particles called atoms. all atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements. the atoms of one element cannot be changed into atoms of a different element by chemical reactions; atoms are. No headers. atomic theory was invented by the ancient greek philosophers leucippus and democritus, who speculated that the world essentially consists of many tiny indivisible particles, which they called atoms, from the greek atomon, meaning ``uncuttable.'' the nature of matter the atomic theory of matter, first postulated by john dalton, is the basis of all modern chemistry.

Chem 1411 Ch 2 Notes Ch 2 Atoms Atomic Structure Subatomic Particle Each element is composed of extremely small particles called atoms. all atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements. the atoms of one element cannot be changed into atoms of a different element by chemical reactions; atoms are. No headers. atomic theory was invented by the ancient greek philosophers leucippus and democritus, who speculated that the world essentially consists of many tiny indivisible particles, which they called atoms, from the greek atomon, meaning ``uncuttable.'' the nature of matter the atomic theory of matter, first postulated by john dalton, is the basis of all modern chemistry. To print or download this file, click the link below: chem 1411. chapter 2. atoms, molecules and ions (homework).with answers.pdf — pdf document, 241 kb (246843 bytes). Chem 1411 general chemistry i (with lab) chapter 2 atoms, molecules, and ions to print or download this file, click the link below: chapter.

Chapter 2 Atoms Molecules And Ions Chem 1411 Chapter 2 Atoms To print or download this file, click the link below: chem 1411. chapter 2. atoms, molecules and ions (homework).with answers.pdf — pdf document, 241 kb (246843 bytes). Chem 1411 general chemistry i (with lab) chapter 2 atoms, molecules, and ions to print or download this file, click the link below: chapter.
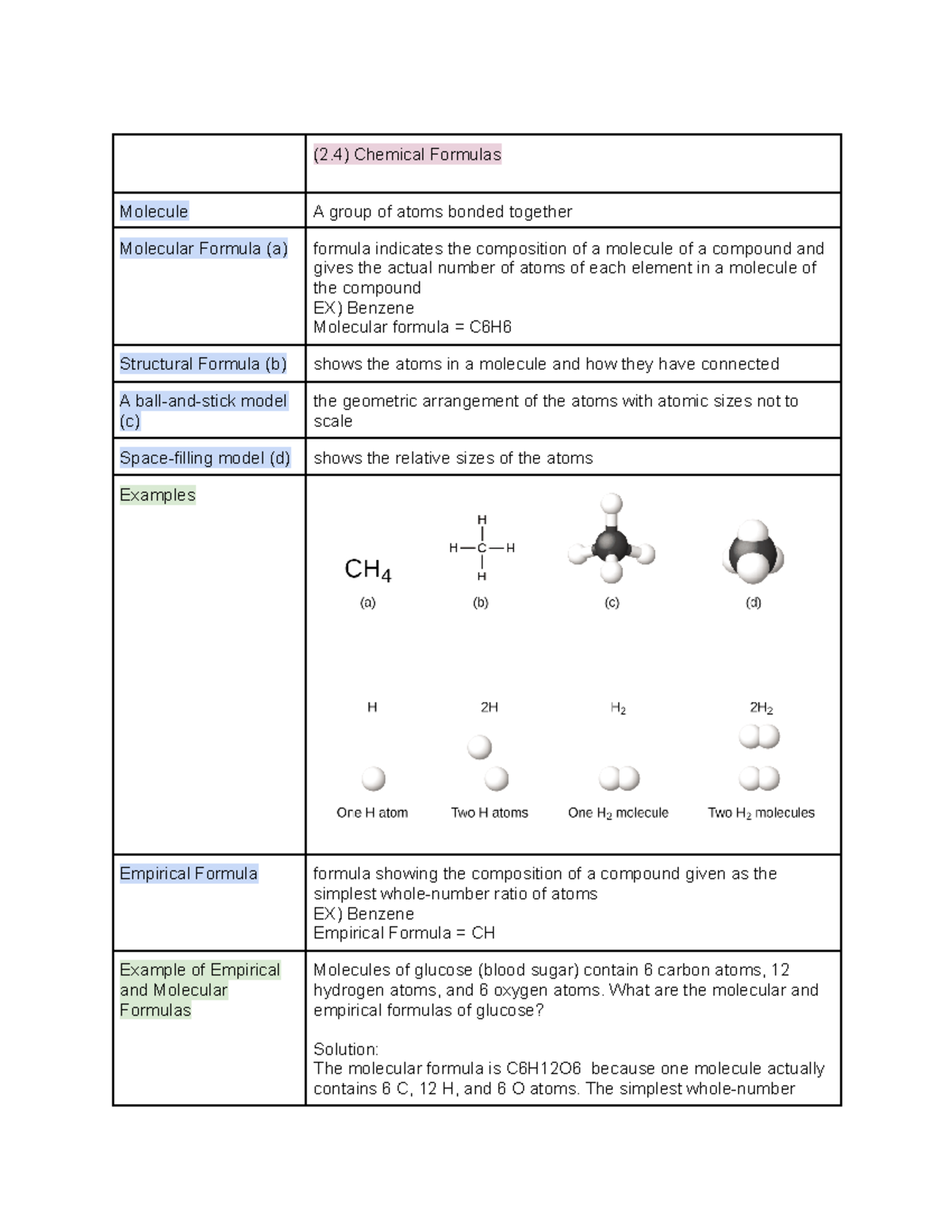
Ch 2 Atoms Molecules And Ions Section 4 Chemical Formulas 2

Chapter 2 Atoms Molecules Ions

Comments are closed.