First New Alzheimer S Drug In 20 Years Approved In The Us Heart
First New Alzheimer S Drug In 20 Years Approved In The Us Heart The first new treatment for alzheimer's disease for nearly 20 years has been approved by regulators in the united states, paving the way for its use in the uk. aducanumab targets the underlying. The food and drug administration has fully approved the first drug shown to slow down alzheimer's disease. the action means that leqembi, whose generic name is lecanemab, should be widely covered.

First New Alzheimer S Drug In 20 Years Approved In The Us Heart The first new drug for alzheimer's disease in nearly 20 years received approval from government health officials on monday. the decision disregarded warnings from independent advisers that the. Lecanemab's approval came with the fda's strongest safety warning, so it's not surprising that the drug's potential side effects are in the spotlight. the most common are headaches and infusion related reactions — nausea, changes in blood pressure, flu like symptoms. "however, in about 15% of patients, amyloid related imaging abnormalities. The us food and drug administration on thursday granted traditional full approval to the alzheimer’s drug leqembi, the first medicine proven to slow the course of the memory robbing disease. For the first time in nearly two decades, the food and drug administration (fda) has approved a drug to slow the progression of the disease. “aducanumab is what’s called a monoclonal antibody, it’s an iv infused manufactured protein that actually is supposed to find the amyloid in the brain and the body and grab it and remove it,” said.
First New Alzheimer S Drug In 20 Years Approved In The Us Heart The us food and drug administration on thursday granted traditional full approval to the alzheimer’s drug leqembi, the first medicine proven to slow the course of the memory robbing disease. For the first time in nearly two decades, the food and drug administration (fda) has approved a drug to slow the progression of the disease. “aducanumab is what’s called a monoclonal antibody, it’s an iv infused manufactured protein that actually is supposed to find the amyloid in the brain and the body and grab it and remove it,” said. Earlier this week, the federal food and drug administration (fda) approved the use of the drug aducanumab to treat alzheimer’s disease – making it the first new drug in nearly 20 years to treat the disease. aducanumab is the first of a new generation of drugs that target the underlying damage in the brain. The first new drugs in more than 20 years for the treatment of alzheimer’s disease are likely to be available here soon. ap. in an 18 month trial involving 1795 people aged 50 to 90, the first.

Fda Approves Aduhelm First New Alzheimer S Drug In 20 Years Youtube Earlier this week, the federal food and drug administration (fda) approved the use of the drug aducanumab to treat alzheimer’s disease – making it the first new drug in nearly 20 years to treat the disease. aducanumab is the first of a new generation of drugs that target the underlying damage in the brain. The first new drugs in more than 20 years for the treatment of alzheimer’s disease are likely to be available here soon. ap. in an 18 month trial involving 1795 people aged 50 to 90, the first.

Excitement As Us Approves First New Alzheimer S Drug In 20 Years
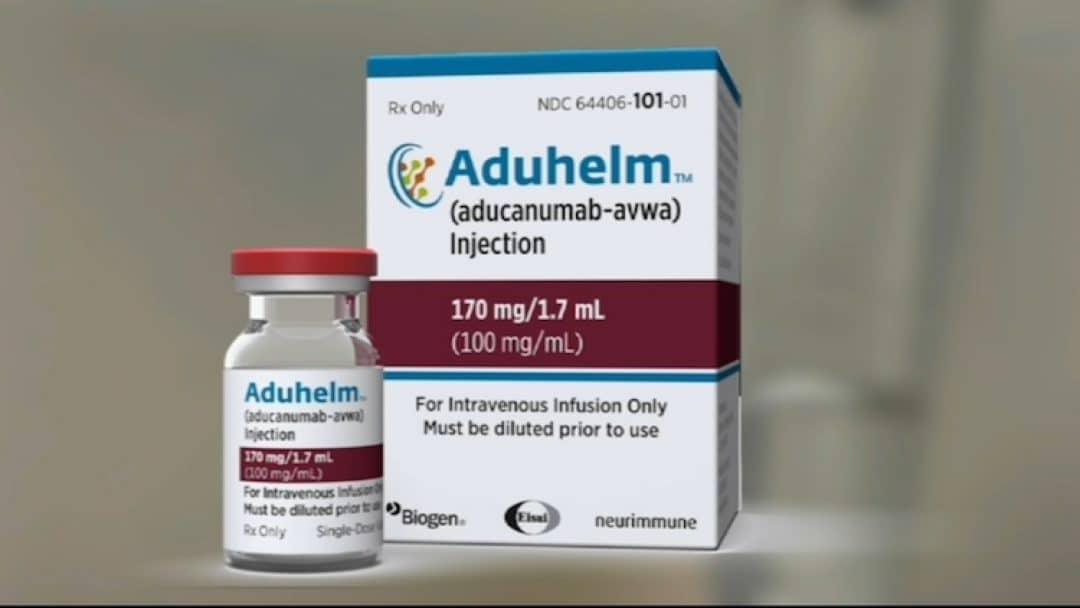
Fda Approves First New Alzheimer S Disease Drug In Nearly 20 Years

Comments are closed.