First Fully Immersive 3d Augmented Reality Surgical Navigation System
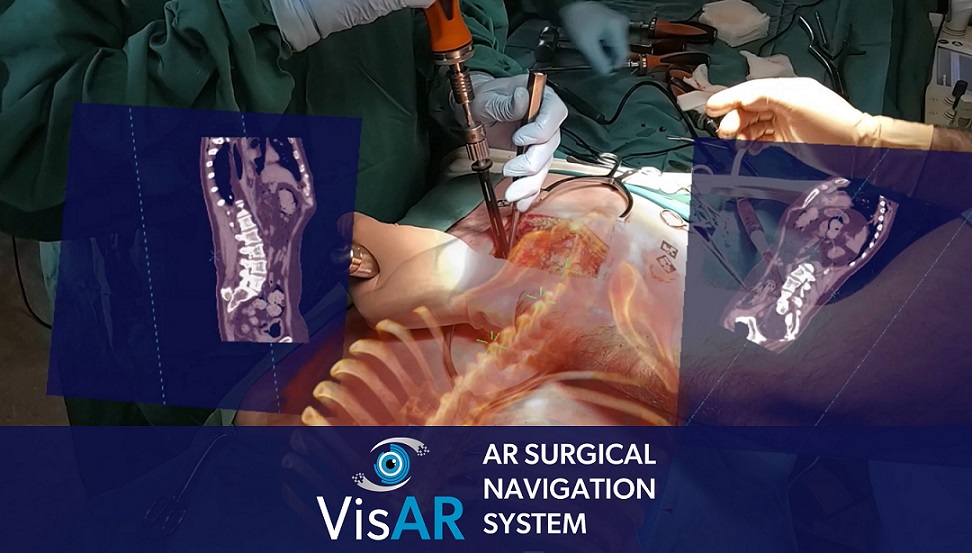
First Fully Immersive 3d Augmented Reality Surgical Navigation System Salt lake city, june 15, 2022 prnewswire visar, an augmented reality surgical navigation system from healthcare technology leader novarad™, receives fda 510(k) approval for precision guided. Salt lake city visar, an augmented reality surgical navigation system from healthcare technology leader novarad™, receives fda 510 (k) approval for precision guided intraoperative spine surgery. visar transforms a patient's imaging data into a 3 dimensional hologram which is visible through an optical visor and superimposed onto the patient.

First Fully Immersive 3d Augmented Reality Surgical Navigation System Salt lake city, june 15, 2022 prnewswire — visar, an augmented reality surgical navigation system from healthcare technology leader novarad™, receives fda 510(k) approval for precision guided intraoperative spine surgery. visar transforms a patient’s imaging data into a 3 dimensional hologram which is visible through an optical visor. "like a surgical gps, visar provides a roadmap to guide the surgeon to the pathology of interest." visar is an end to end solution with pre surgical planning, virtual annotations, segmentation and bi directional image connectivity. it features integrated 2d and 3d immersive navigation views with continuous hologram to patient registration. Immersivetouch wins fda clearance for breakthrough augmented reality surgical system immersivear™ officially brings precision enhanced visualization into the operating room july 10, 2024 01:00. Immersivear™ officially brings precision enhanced visualization into the operating room. chicago, july 10, 2024–(business wire)–immersivetouch inc., a leading digital surgery company, announced today that its groundbreaking augmented reality (ar) technology platform, immersivear, has received 510(k) clearance for clinical use in the operating room by the u.s. food and drug administration.

First Fully Immersive 3d Augmented Reality Surgical Navigation System Immersivetouch wins fda clearance for breakthrough augmented reality surgical system immersivear™ officially brings precision enhanced visualization into the operating room july 10, 2024 01:00. Immersivear™ officially brings precision enhanced visualization into the operating room. chicago, july 10, 2024–(business wire)–immersivetouch inc., a leading digital surgery company, announced today that its groundbreaking augmented reality (ar) technology platform, immersivear, has received 510(k) clearance for clinical use in the operating room by the u.s. food and drug administration. Visualisation augmented reality (visar) is an ar surgical navigation system developed by us based medical device company novarad. it is designed to assist surgeons find the precise location of anatomical structures in either open or percutaneous spine procedures. the system smoothly incorporates ar into the surgeon’s line of sight. Immersive augmented reality surgical navigation . visar is a next generation surgical navigation system with fda 510(k) clearance for precision guided intraoperative spinal surgery, with other fda clearances pending. it provides the precision of a robot, the portability of a stethoscope, and the versatility of human powered intelligence.

Comments are closed.