Finding Theoretical Yield And Percent Yield

How To Calculate The Percent Yield And Theoretical Yield Youtube The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100 to give a percentage: percent yield = actual yield (g) theoretical yield(g) × 100%. the method used to calculate the percent yield of a reaction is illustrated in example 4.3.4. The percent yield is determined by calculating the ratio of actual yield to theoretical yield. this page is shared under a ck 12 license and was authored, remixed, and or curated by melissa alviar agnew, henry agnew, vicki macmurdo (anoka ramsey community college), and lance s. lund (anoka ramsey community college).

How To Calculate Theoretical Yield And Percent Yield Youtube Determine the theoretical yield: use stoichiometry to find the theoretical yield, ensuring it is in the same units as the actual yield. measure the actual yield: obtain this from your experimental data. apply the percent yield formula: insert your actual and theoretical yields into the percent yield formula to find the efficiency of your reaction. Limiting reactant. percent yield. theoretical yield. 6.2: limiting reactant, theoretical yield, and percent yield is shared under a not declared license and was authored, remixed, and or curated by libretexts. when reactions are carried out using less than stoichiometric quantities of reactants, the amount of product generated will be. 5. convert the result to grams. this is the reverse of your earlier step of calculating the number of moles or reactant. when you know the number of moles that you expect, you will multiply by the molar mass of the product to find the theoretical yield in grams. Find the moles of the limiting reagent. multiply the moles of the limiting reagent by the stoichiometry of carbon dioxide in the reaction to give the moles of co 2 produced. multiply the moles of co 2 produced by 44, the molecular weight of co 2, to get the theoretical yield of your reaction.
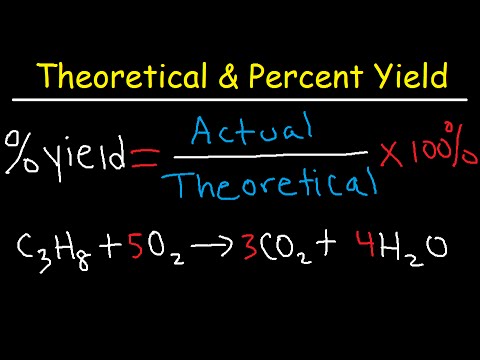
How To Calculate Theoretical Yield And Percent Yield Youtube 5. convert the result to grams. this is the reverse of your earlier step of calculating the number of moles or reactant. when you know the number of moles that you expect, you will multiply by the molar mass of the product to find the theoretical yield in grams. Find the moles of the limiting reagent. multiply the moles of the limiting reagent by the stoichiometry of carbon dioxide in the reaction to give the moles of co 2 produced. multiply the moles of co 2 produced by 44, the molecular weight of co 2, to get the theoretical yield of your reaction. To calculate the percent yield, we take the actual yield and divide it by the theoretical yield and multiply by 100: 65.2 g zn(no 3) 2 88.3 zn(no 3) 2 × 100 % = 73.8 % the worker achieved almost three fourths of the possible yield. test yourself. a synthesis produced 2.05 g of nh 3 from 16.5 g of n 2. what is the theoretical yield and the. Percent yield = actual yield theoretical yield × 100 % percent yield = actual yield theoretical yield × 100 % actual and theoretical yields may be expressed as masses or molar amounts (or any other appropriate property; e.g., volume, if the product is a gas).

Comments are closed.