Fda Recommends New Dosing Instructions

Pdf Current Fda Related Drug Information New Drugs Approved By The The u.s. food and drug administration (fda) is notifying the public that fda has approved label changes specifying new dosing recommendations for zolpidem products (ambien, ambien cr, and edluar. The u.s. food and drug administration (fda) is notifying the public of new information about zolpidem, a widely prescribed insomnia drug. fda recommends that the bedtime dose be lowered because.
Draft Fda Labeling Guidance Proposes More Clarity For Providers Until vaccine manufacturers have data and science supporting a change, we continue to strongly recommend that health care providers follow the fda authorized dosing schedule for each covid 19 vaccine. Precision drug dosing recommended actions over the investigational new drug (ind) and new drug application (nda) life cycle. there are eight proposed actions over the research and development drug cycle designed to produce more precise dosing recommendations at market approval or soon thereafter and continuously improve dosing based on real. The recommended vaccine and number of 2024–2025 covid 19 vaccine doses are based on age and vaccination history. covid 19 vaccination guidance for people who are moderately or severely immunocompromised is detailed in table 2. table 1. routine covid 19 vaccination schedule, october 31, 2024. Do not inject wegovy into a muscle (intramuscularly) or vein (intravenously). • change (rotate) your injection site with each injection. do not use the same site for each injection. • use wegovy 1 time each week, on the same day each week, at any time of the day. • start wegovy with 0.25 mg per week in your first month.
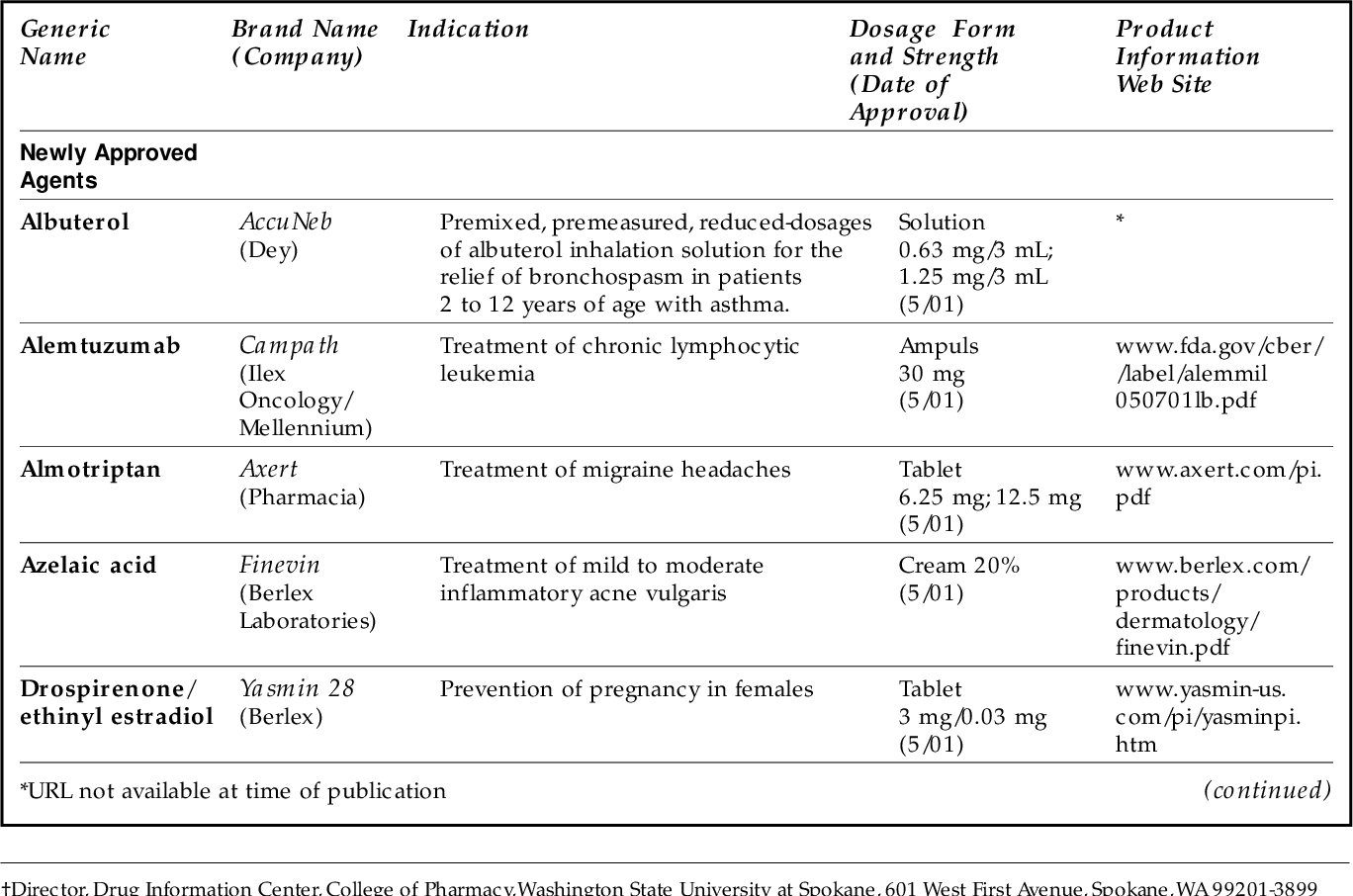
Table 1 From New Drugs Approved By The Fda New Dosage Forms And The recommended vaccine and number of 2024–2025 covid 19 vaccine doses are based on age and vaccination history. covid 19 vaccination guidance for people who are moderately or severely immunocompromised is detailed in table 2. table 1. routine covid 19 vaccination schedule, october 31, 2024. Do not inject wegovy into a muscle (intramuscularly) or vein (intravenously). • change (rotate) your injection site with each injection. do not use the same site for each injection. • use wegovy 1 time each week, on the same day each week, at any time of the day. • start wegovy with 0.25 mg per week in your first month. Paxil is not approved for use in pediatric patients. (5.1, 8.4) elderly patients, patients with severe renal impairment or severe hepatic impairment: starting dosage is 10 mg daily. maximum dosage is 40 mg daily. (2.4) extended release tablets: 10 mg, scored; 20 mg, scored; 30 mg; and 40 mg tablets. Recommended dose of zolpidem for women should be lowered from 10 mg to 5rmg fo immediate‐release products (ambien, edluar, and zolpimist) and from 12.5 mg to 6.25 mg for extended‐release.
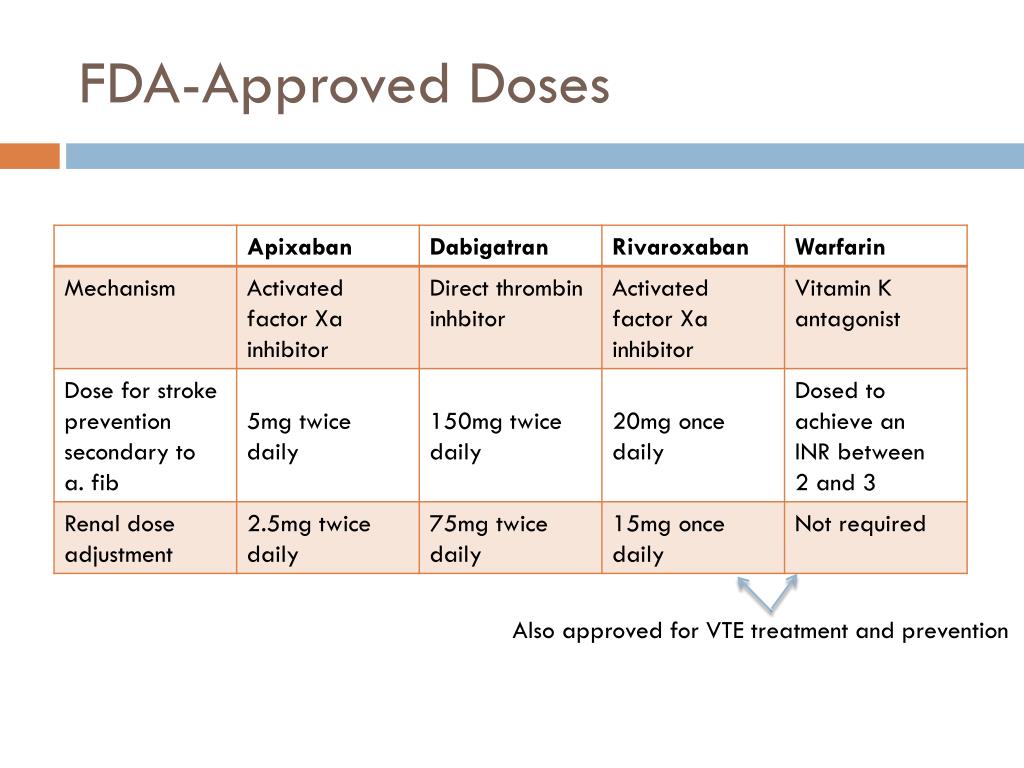
Ppt Oral Anticoagulants In The 21 St Century A Practical Guide To Paxil is not approved for use in pediatric patients. (5.1, 8.4) elderly patients, patients with severe renal impairment or severe hepatic impairment: starting dosage is 10 mg daily. maximum dosage is 40 mg daily. (2.4) extended release tablets: 10 mg, scored; 20 mg, scored; 30 mg; and 40 mg tablets. Recommended dose of zolpidem for women should be lowered from 10 mg to 5rmg fo immediate‐release products (ambien, edluar, and zolpimist) and from 12.5 mg to 6.25 mg for extended‐release.

New Dosage Forms And Indications Approved By The Fda February 18 2013

Covid 19 Vaccine Receives Full Fda Approval

Comments are closed.