Fda Approval And Dosing Of Currently Approved Ivi Formulations

Fda Approval And Dosing Of Currently Approved Ivi Formulations Download table | fda approval and dosing of currently approved ivi formulations from publication: considerations and challenges in defining optimal iron utilization in hemodialysis | trials. With this approval of cosentyx as an iv formulation, along with the subcutaneous formulation, we can broaden the use of cosentyx to help more patients manage their condition with a medicine backed.

Fda Approval And Dosing Of Currently Approved Ivi Formulations The intravenous formulation of cosentyx® was approved by the fda on october 7, 2023. permanent j code7 j3247 package strength1 125 mg 5 ml (25 mg ml) solution in a single dose vial for dilution prior to intravenous infusion description7 injection, secukinumab, intravenous, 1 mg billing unit 1 unit per 1 mg ndc1 10 digit: 0078 1168 61 11 digit. The fda also approved a new capsule dosage form available in strengths of 50 mg and 100 mg. 9 26 2023 fda approves new and updated indications for temozolomide under project renewal. Docetaxel is the first line of chemotherapeutic among taxane group of drugs which was approved by the us fda in 1996 for the treatment of breast cancer (falvo et al., 2021). used at the dosage of 60 mg m 2 to 100 mg m 2 as a single agent and in 75 mg m 2 in combination with doxorubicin and cyclophosphamide. Previously approved drug products were adequate by current standards. if this is not the case, and the change in formulation or route of administration triggers the need for additional studies,.
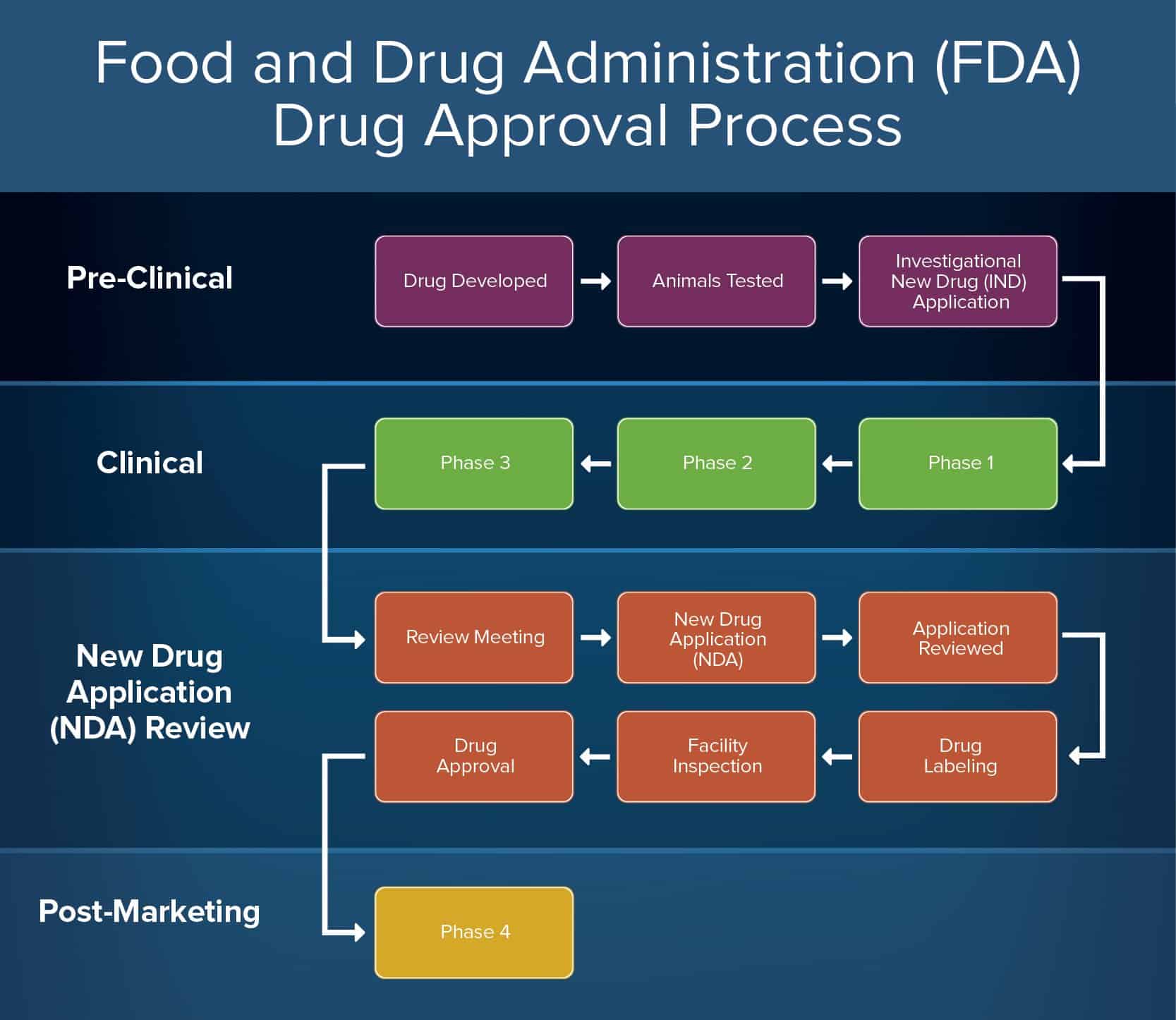
How To Create An Approval Process Smartsheet Docetaxel is the first line of chemotherapeutic among taxane group of drugs which was approved by the us fda in 1996 for the treatment of breast cancer (falvo et al., 2021). used at the dosage of 60 mg m 2 to 100 mg m 2 as a single agent and in 75 mg m 2 in combination with doxorubicin and cyclophosphamide. Previously approved drug products were adequate by current standards. if this is not the case, and the change in formulation or route of administration triggers the need for additional studies,. A total of 206 novel drugs in tablet or capsule formulation were approved by the fda between 1995 and 2010 . eighty one (39.3%) were followed by at least 1 new fda approved formulation (figure 1 and efigure 2 in the supplement), and 167 (81.1%) had at least 1 generic version approved by the fda as of december 31, 2021 . among the 206 novel. Injection: 80 mg 4 ml (20 mg ml), 200 mg 10 ml (20 mg ml), 400 mg 20 ml (20 mg ml) in single dose vials for further dilution prior to . intravenous infusion (3) subcutaneous injection injection: 162 mg 0.9 ml in a single dose prefilled syringe or single dose prefilled actpen ® autoinjector (3) reference id: 4757547 . 1.

Currently Fda Approved Adcs Dosing Schedules And Key Information A total of 206 novel drugs in tablet or capsule formulation were approved by the fda between 1995 and 2010 . eighty one (39.3%) were followed by at least 1 new fda approved formulation (figure 1 and efigure 2 in the supplement), and 167 (81.1%) had at least 1 generic version approved by the fda as of december 31, 2021 . among the 206 novel. Injection: 80 mg 4 ml (20 mg ml), 200 mg 10 ml (20 mg ml), 400 mg 20 ml (20 mg ml) in single dose vials for further dilution prior to . intravenous infusion (3) subcutaneous injection injection: 162 mg 0.9 ml in a single dose prefilled syringe or single dose prefilled actpen ® autoinjector (3) reference id: 4757547 . 1.

Currently Fda Approved Adcs Dosing Schedules And Key Information

Comments are closed.