Expert Perspectives On Covid 19 Vaccination For People Living With
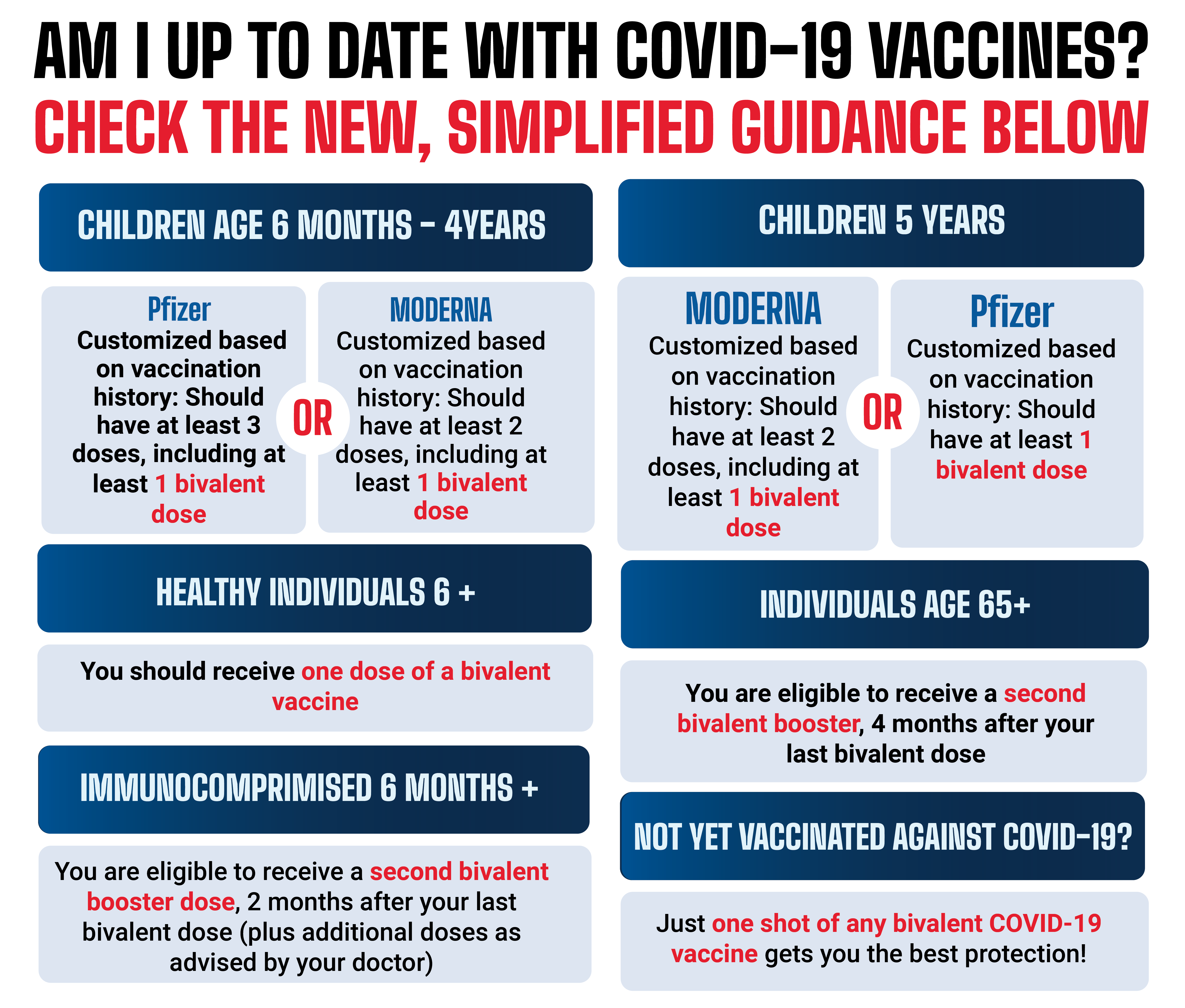
Vaccine Basics Covid19 Covid 19 vaccines are safe for people living with multiple sclerosis (ms) on or off disease modifying therapies (dmts) and are important for the prevention of coronavirus disease 2019 (covid 19). antibody responses for individuals on certain dmts may be diminished, however, t cell responses may be preserved in those individuals. The once promising pace of covid 19 vaccination in the united states has slowed, from a peak of 3.38 million shots on april 13, 2021, to fewer than 2 million doses per day in may.
.jpg?h=1d0e8fe7&itok=o5KS_Y-Y)
Covid Vaccine Georgia Department Of Public Health Community‐based studies in five countries show consistent strong benefits from early rollouts of covid‐19 vaccines. by the beginning of june 2021, almost 11% of the world’s population had received at least one dose of a coronavirus disease 2019 (covid‐19) vaccine. 1 this represents an extraordinary scientific and logistic achievement — in 18 months, researchers, manufacturers and. Prior surveys 15,16,17 have examined preferences regarding scarce resource allocation in the pandemic, but few have focused specifically on covid 19 vaccine allocation. to address this gap, we surveyed 2 representative samples of us adults about covid 19 vaccine allocation priorities. our study builds on prior surveys in key ways. The vaccines bnt162b2 (brand name comirnaty), mrna 1273 (brand name spikevax), coronavac, bbibp corv, azd 1222 (brand name vaxzevria or covishield), and ad26.cov2 s (brand name janssen covid 19 vaccine) are the most widely used around the world for covid 19 prophylaxis, since all of them use the s protein as the main activator of the immune. The justification often is—and certainly is the case with these early covid vaccines—that we don’t know enough yet about the vaccine or the vaccine platform or the safety of the vaccine to do a study in pregnant people. with the mrna vaccine, for example, [the type of vaccine being considered for covid 19] we don’t currently have a.
Unc Immunology Expert Discusses Covid 19 Vaccines Debunks Misinformation The vaccines bnt162b2 (brand name comirnaty), mrna 1273 (brand name spikevax), coronavac, bbibp corv, azd 1222 (brand name vaxzevria or covishield), and ad26.cov2 s (brand name janssen covid 19 vaccine) are the most widely used around the world for covid 19 prophylaxis, since all of them use the s protein as the main activator of the immune. The justification often is—and certainly is the case with these early covid vaccines—that we don’t know enough yet about the vaccine or the vaccine platform or the safety of the vaccine to do a study in pregnant people. with the mrna vaccine, for example, [the type of vaccine being considered for covid 19] we don’t currently have a. The experience with influenza a and b vaccines offers a perspective for covid 19 vaccines when transmission is associated with the capacity of the target virus to undergo seasonal antigenic drift. Pivotal studies have shown that vaccination is one of the effective ways to prevent severe covid 19 illness in the general population. studies on people living with hiv (plwh) are scarce. the majority of these studies with mrna (bnt126b2 and mrna 1273) and adenovirus vector (ad26.cov2.2 and chadox1) vaccines with a low number of patients.

Covid 19 Vaccine S Effectiveness Diminishes With Age Utsw Research The experience with influenza a and b vaccines offers a perspective for covid 19 vaccines when transmission is associated with the capacity of the target virus to undergo seasonal antigenic drift. Pivotal studies have shown that vaccination is one of the effective ways to prevent severe covid 19 illness in the general population. studies on people living with hiv (plwh) are scarce. the majority of these studies with mrna (bnt126b2 and mrna 1273) and adenovirus vector (ad26.cov2.2 and chadox1) vaccines with a low number of patients.
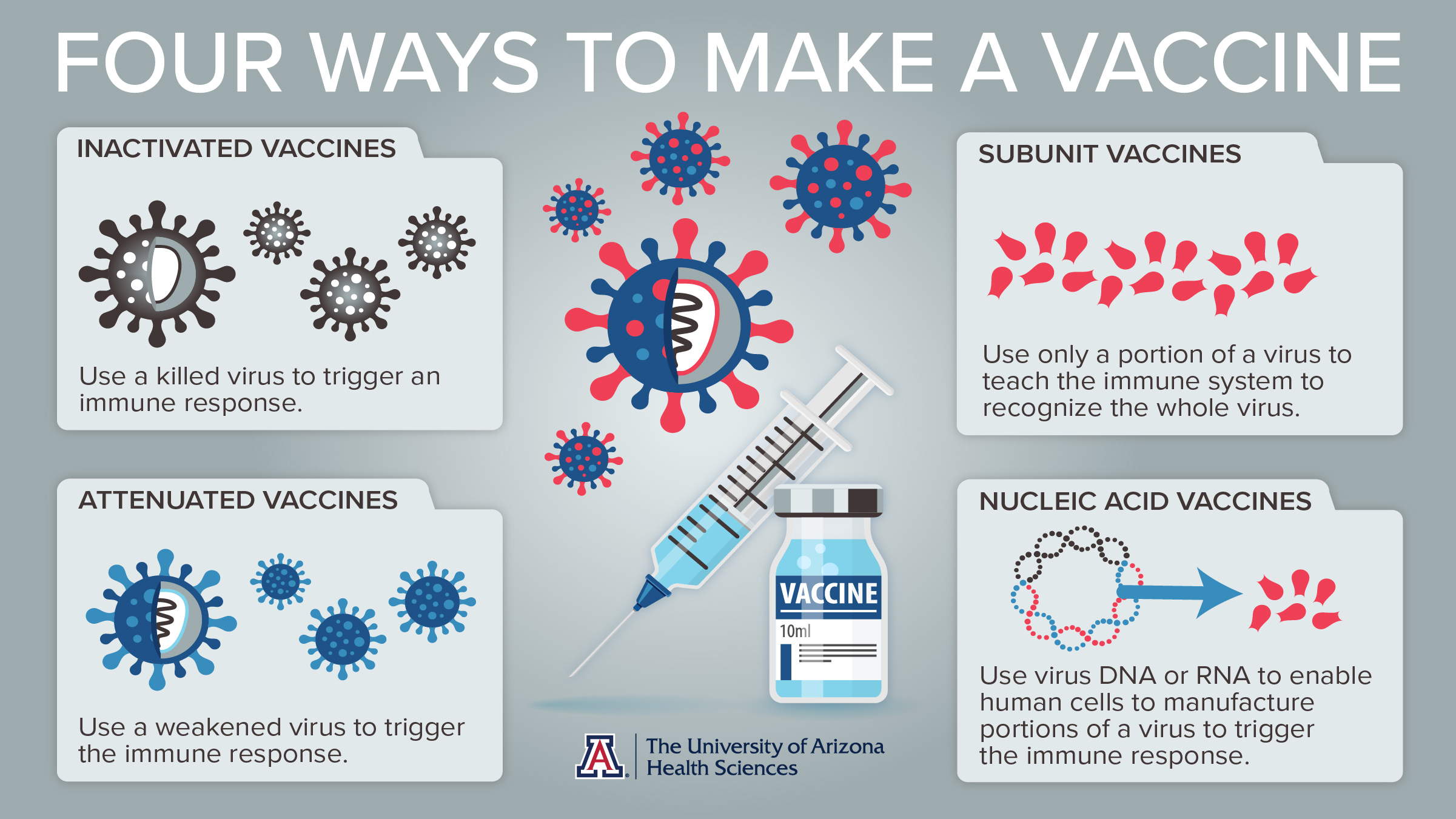
What It Takes To Create A Vaccine The University Of Arizona Health
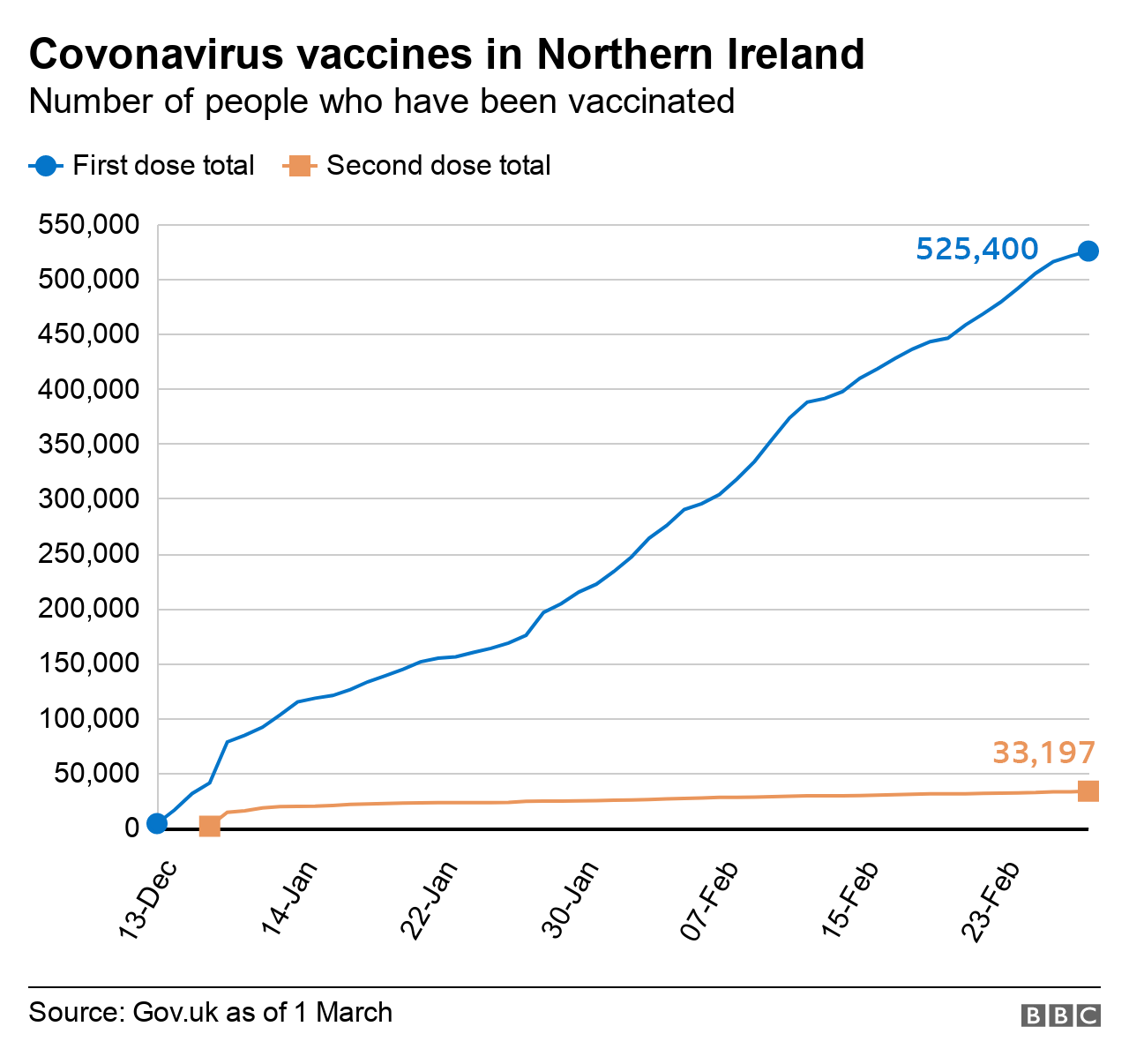
Covid 19 Vaccines Extended To People Aged 60 To 64 Bbc News

Comments are closed.