Energy Work And Heat Youtube
Thermodynamics Energy Work And Heat Animation Youtube This chemistry video tutorial provides a basic introduction into internal energy, heat, and work as it relates to thermodynamics. it explains how to calcula. In chemistry we talked about the first law of thermodynamics as being the law of conservation of energy, and that's one way of looking at it, but physicists.

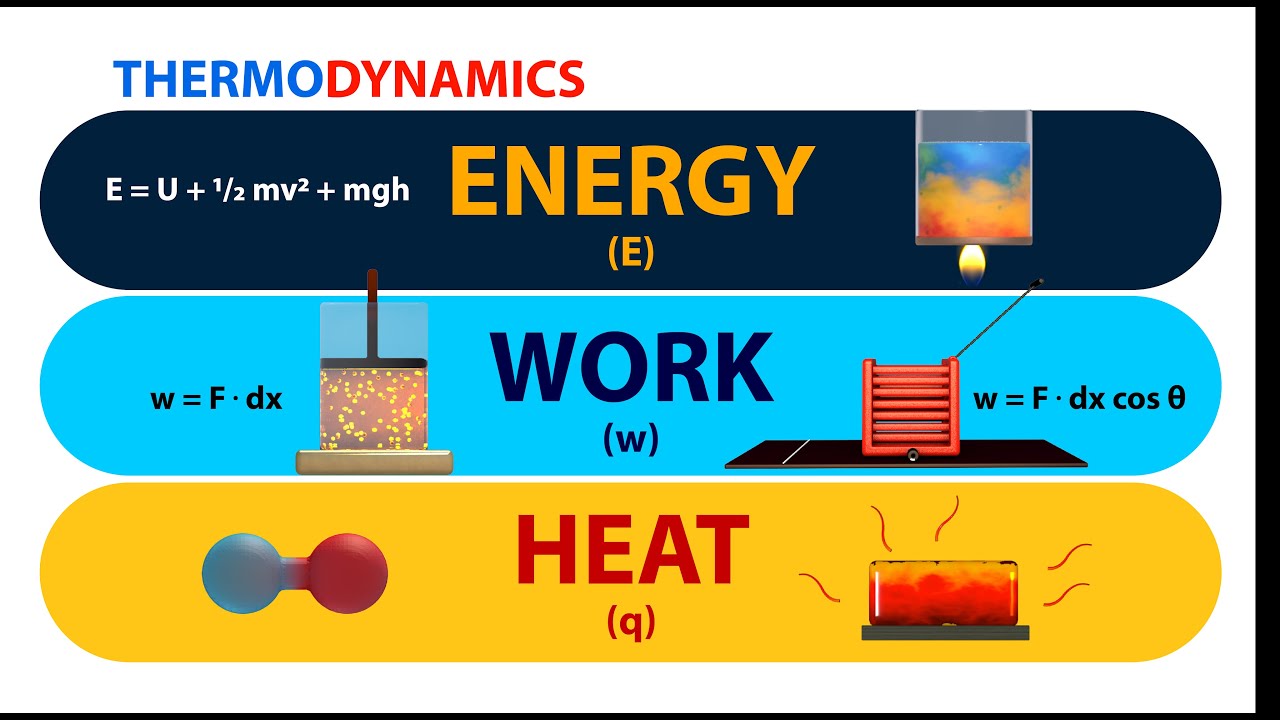
Energy Work And Heat Youtube This chemistry video tutorial provides a basic introduction into the first law of thermodynamics. it shows the relationship between internal energy, heat, a. The maximum amount of work one can attain from heat is given by the carnot efficiency. heat is the energy associated with the random motion of particles, while work is the energy of ordered motion in one direction. therefore heat is "low quality" energy and work is "high quality" energy, and this supports the entropy statement of the second law. Energy is transferred along with the genetic material and so obeys the first law of thermodynamics. energy is transferred—not created or destroyed—in the process. when work is done on a cell or heat transfers energy to a cell, the cell’s internal energy increases. when a cell does work or loses heat, its internal energy decreases. This page titled 3.3: work, heat, and internal energy is shared under a cc by 4.0 license and was authored, remixed, and or curated by openstax via source content that was edited to the style and standards of the libretexts platform. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure.

Energy Work And Heat Youtube Energy is transferred along with the genetic material and so obeys the first law of thermodynamics. energy is transferred—not created or destroyed—in the process. when work is done on a cell or heat transfers energy to a cell, the cell’s internal energy increases. when a cell does work or loses heat, its internal energy decreases. This page titled 3.3: work, heat, and internal energy is shared under a cc by 4.0 license and was authored, remixed, and or curated by openstax via source content that was edited to the style and standards of the libretexts platform. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. Summary. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. heat is the energy transferred between two objects (or two parts of a system) because of a temperature difference. internal energy of a thermodynamic system is its total mechanical energy. Energy, heat and work. energy is the ability to move an object against a resisting force. moving an object against a resisting force is called work, so we can write our energy definition more formally: energy is the ability to do work. work is a way to transfer energy from one collection of matter to another.

Relation Between Internal Energy Work And Heat Youtube Summary. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. heat is the energy transferred between two objects (or two parts of a system) because of a temperature difference. internal energy of a thermodynamic system is its total mechanical energy. Energy, heat and work. energy is the ability to move an object against a resisting force. moving an object against a resisting force is called work, so we can write our energy definition more formally: energy is the ability to do work. work is a way to transfer energy from one collection of matter to another.

Chapter 1c Introduction To Energy Work And Heat Youtube

Heat Energy Work Youtube

Comments are closed.