Energy Work And Heat
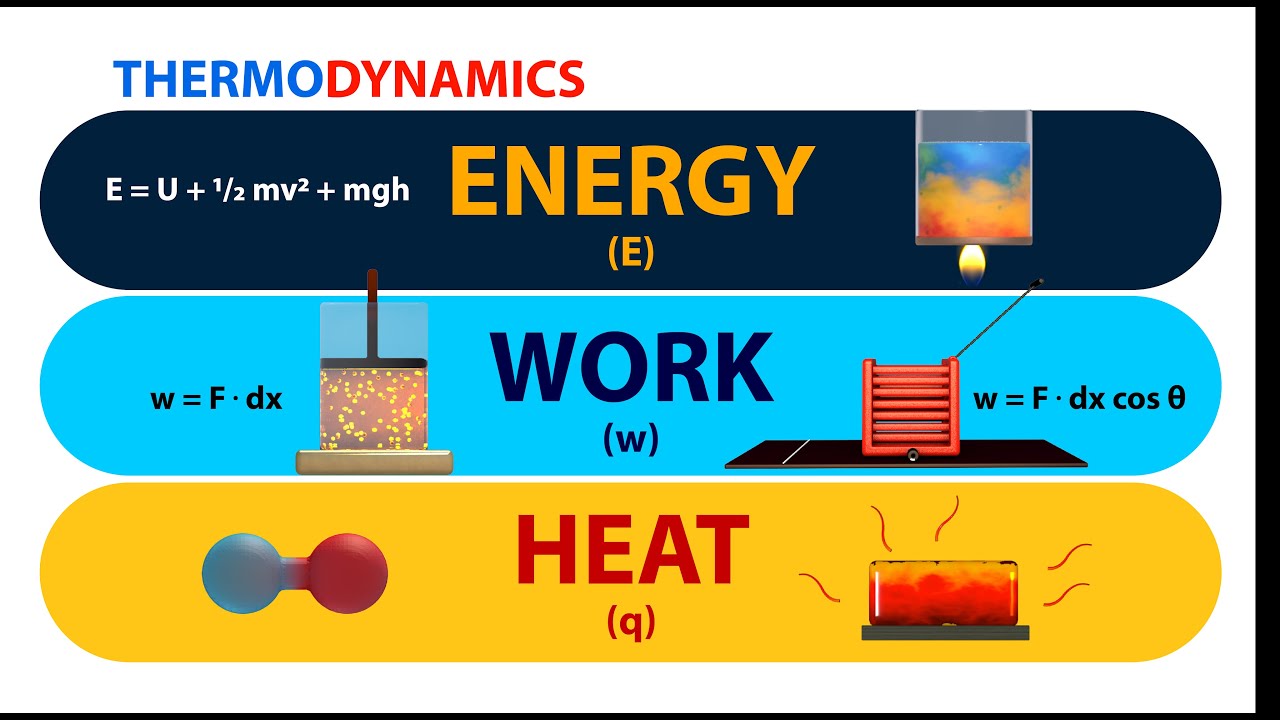
Thermodynamics Energy Work And Heat Animation Youtube This page titled 3.3: work, heat, and internal energy is shared under a cc by 4.0 license and was authored, remixed, and or curated by openstax via source content that was edited to the style and standards of the libretexts platform. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. Note that if heat transfers more energy into the system than that which is done by work, the difference is stored as internal energy. figure 12.6 the first law of thermodynamics is the conservation of energy principle stated for a system, where heat and work are the methods of transferring energy to and from a system.

Energy Work And Heat Youtube The maximum amount of work one can attain from heat is given by the carnot efficiency. heat is the energy associated with the random motion of particles, while work is the energy of ordered motion in one direction. therefore heat is "low quality" energy and work is "high quality" energy, and this supports the entropy statement of the second law. Energy, heat and work. energy is the ability to move an object against a resisting force. moving an object against a resisting force is called work, so we can write our energy definition more formally: energy is the ability to do work. work is a way to transfer energy from one collection of matter to another. Energy is measured in terms of its ability to perform work or to transfer heat. mechanical work is done when a force f displaces an object by a distance d: w = f × d. the basic unit of energy is the joule. one joule is the amount of work done when a force of 1 newton acts over a distance of 1 m; thus 1 j = 1 n m. Thermodynamics is the science of the relationship between heat, work, temperature, and energy. heat was not formally recognized as a form of energy until about 1798, when count rumford (sir benjamin thompson), a british military engineer, noticed that limitless amounts of heat could be generated in the boring of cannon barrels and that the.

Energy Heat Work And Thermodynamic Processes Energy is measured in terms of its ability to perform work or to transfer heat. mechanical work is done when a force f displaces an object by a distance d: w = f × d. the basic unit of energy is the joule. one joule is the amount of work done when a force of 1 newton acts over a distance of 1 m; thus 1 j = 1 n m. Thermodynamics is the science of the relationship between heat, work, temperature, and energy. heat was not formally recognized as a form of energy until about 1798, when count rumford (sir benjamin thompson), a british military engineer, noticed that limitless amounts of heat could be generated in the boring of cannon barrels and that the. If energy is transferred from your hands to the object, your hands feel cold. because heat is a measure of energy transfer, heat is also measured in joules. for a given object, the amount of heat (q) involved is proportional to two things: the mass of the object (\(m\)) and the temperature change (\(Δt\)) evoked by the energy transfer. we can. Physical chemistry (essentials) class 11. course: physical chemistry (essentials) class 11 > unit 6. lesson 2: first law of thermodynamics. first law of thermodynamics introduction. more on internal energy. calculating internal energy and work example. heat and temperature.
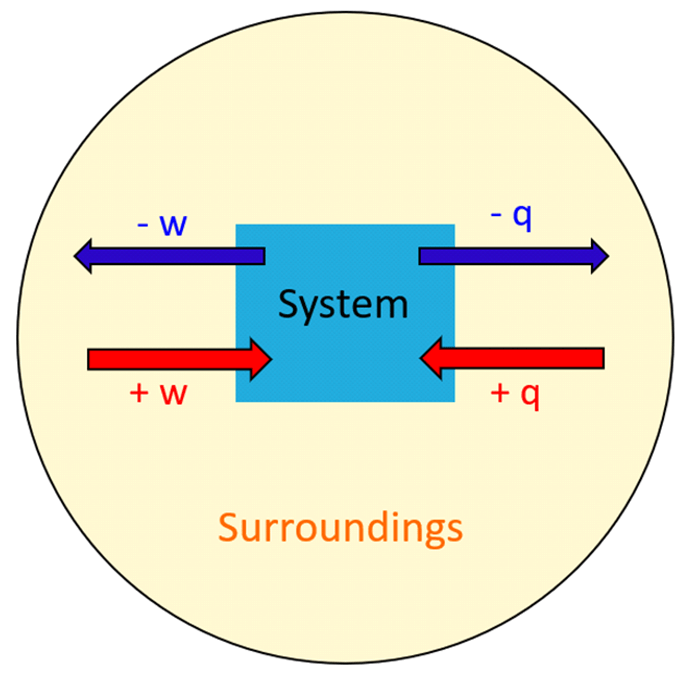
Endothermic And Exothermic Processes Chemistry Steps If energy is transferred from your hands to the object, your hands feel cold. because heat is a measure of energy transfer, heat is also measured in joules. for a given object, the amount of heat (q) involved is proportional to two things: the mass of the object (\(m\)) and the temperature change (\(Δt\)) evoked by the energy transfer. we can. Physical chemistry (essentials) class 11. course: physical chemistry (essentials) class 11 > unit 6. lesson 2: first law of thermodynamics. first law of thermodynamics introduction. more on internal energy. calculating internal energy and work example. heat and temperature.

Comments are closed.