Energy Of Photons Example
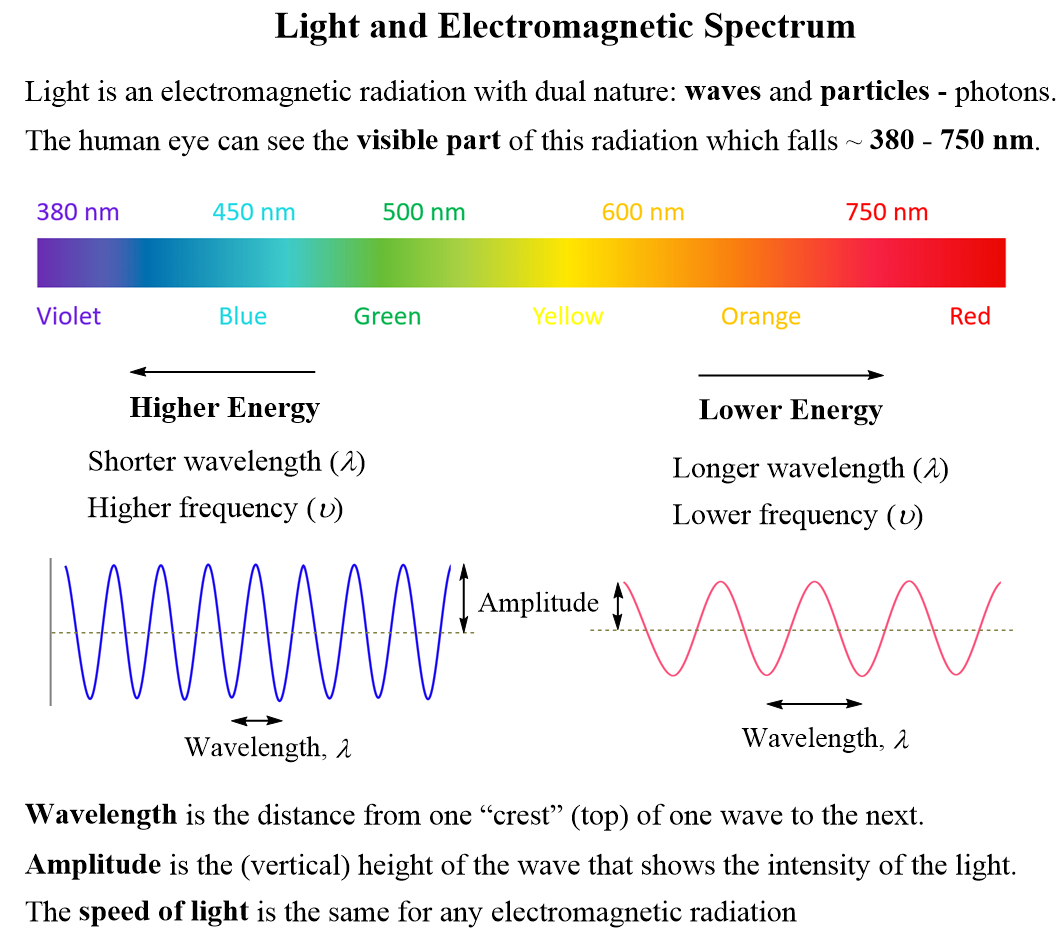
Calculating The Energy Of A Photon Chemistry Steps Calculating the number of photons. example: calculate the number of photons in a laser pulse with a wavelength of 537 nm that contains 6.29 mj (milli joules) of energy. solution: remember, the formula correlating the energy and the wavelength is for one photon. therefore, we can calculate the energy of one photon for the light of 537 nm wavelength. Figure 29.3.1 29.3. 1: the em spectrum, showing major categories as a function of photon energy in ev, as well as wavelength and frequency. certain characteristics of em radiation are directly attributable to photon energy alone. photons act as individual quanta and interact with individual electrons, atoms, molecules, and so on.
How To Calculate The Energy Of A Photon Physics Study Photon energy. photon energy is the energy carried by a single photon. the amount of energy is directly proportional to the photon's electromagnetic frequency and thus, equivalently, is inversely proportional to the wavelength. the higher the photon's frequency, the higher its energy. equivalently, the longer the photon's wavelength, the lower. F =c λ. where c is the speed of light, f the frequency and λ the wavelength. if you know the frequency, or if you just calculated it, you can find the energy of the photon with planck's formula: e = h × f. where h is the planck's constant: h = 6.62607015e 34 m² · kg s. 3. remember to be consistent with the units!. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. for example, if a red photon of frequency f f encounters a molecule that has an energy step, Δ e, Δ e, equal to hf, hf, then the photon can be absorbed. violet flowers absorb red and reflect violet; this implies there. The energy of a photon is given by the equation e = h ν, where 'e' is the photon energy, 'h' is planck's constant and 'ν' is the frequency of the associated light. this equation is also known as planck's formula. planck's constant (h) is a fundamental quantum effects scale, approximately valued at 6.63 × 10 − 34 j s.

Calculate Photon Energy Using Light Wavelength Equation Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. for example, if a red photon of frequency f f encounters a molecule that has an energy step, Δ e, Δ e, equal to hf, hf, then the photon can be absorbed. violet flowers absorb red and reflect violet; this implies there. The energy of a photon is given by the equation e = h ν, where 'e' is the photon energy, 'h' is planck's constant and 'ν' is the frequency of the associated light. this equation is also known as planck's formula. planck's constant (h) is a fundamental quantum effects scale, approximately valued at 6.63 × 10 − 34 j s. The above formula is applicable to a single photon. when more photons are emitted, consider n number of photons, then the formula is given by: e = n × h × f. energy is calculated in joules and electronvolt (ev), depending on the system of the unit used. 1 joule = 6.24 × 10 18 ev. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. for example, if a red photon of frequency f encounters a molecule that has an energy step, Δe, equal to hf, then the photon can be absorbed. violet flowers absorb red and reflect violet; this implies there is no energy.
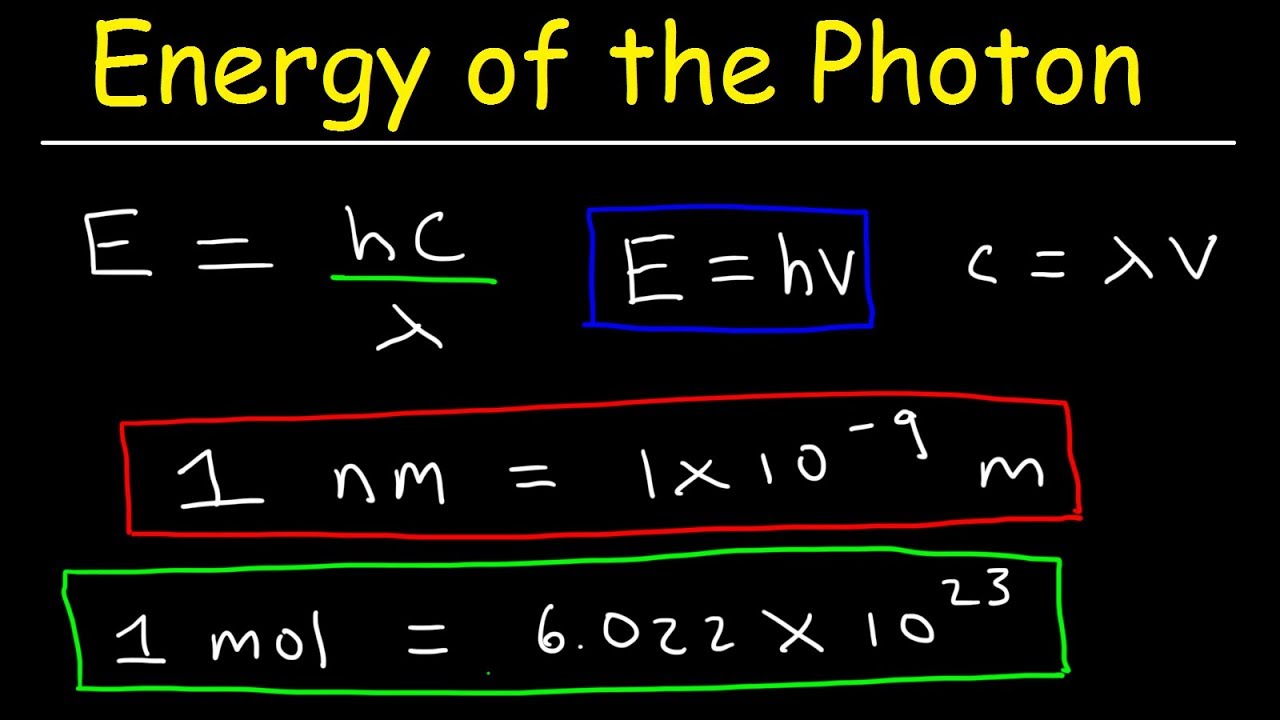
How To Calculate The Energy Of A Photon Given Frequency Wavelength In The above formula is applicable to a single photon. when more photons are emitted, consider n number of photons, then the formula is given by: e = n × h × f. energy is calculated in joules and electronvolt (ev), depending on the system of the unit used. 1 joule = 6.24 × 10 18 ev. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. for example, if a red photon of frequency f encounters a molecule that has an energy step, Δe, equal to hf, then the photon can be absorbed. violet flowers absorb red and reflect violet; this implies there is no energy.
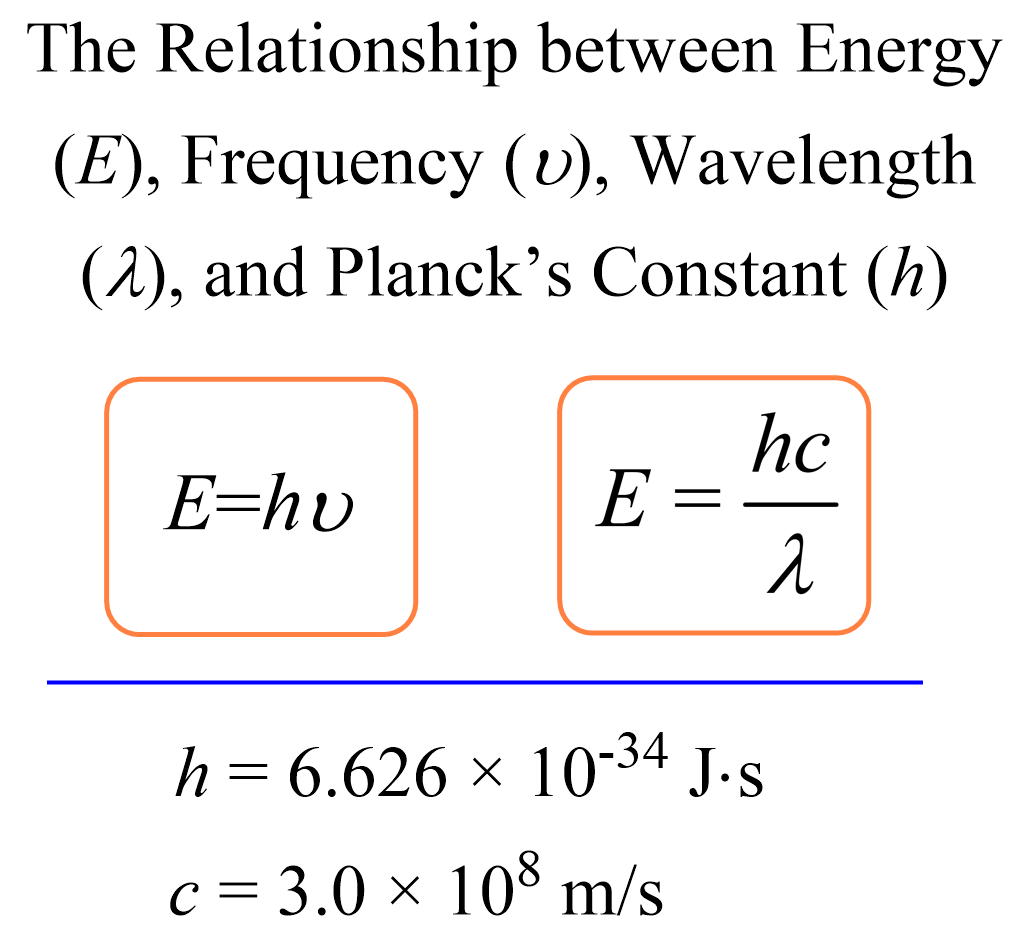
Calculating The Energy Of A Photon Chemistry Steps

Comments are closed.