Dr Subbiah Presents Encouraging Fgfr Inhibitor From The Fight 101 Trial

Vivek Subbiah Md On Twitter Trials In Progress Session Asco Asco21 Pemigatinib safe, demonstrates encouraging responses among patients with advanced malignancies and an fgfr mutation: fight 101 trial. vivek subbiah, md, university of texas md anderson cancer center, houston, overviews the fight 101 trial on pemigatinib, a selective fgfr 1 3 inhibitor, which targets mutations across a variety of tumor types. Pemigatinib, a potent and selective fgfr 1–3 inhibitor, was safe and tolerable and demonstrated pharmacologic clinical activity in cholangiocarcinoma, urothelial carcinoma, recurrent pilocytic astrocytoma, head and neck, pancreatic, gallbladder, uterine, and non small cell lung cancers. the results supported those of fight 202 that led to.
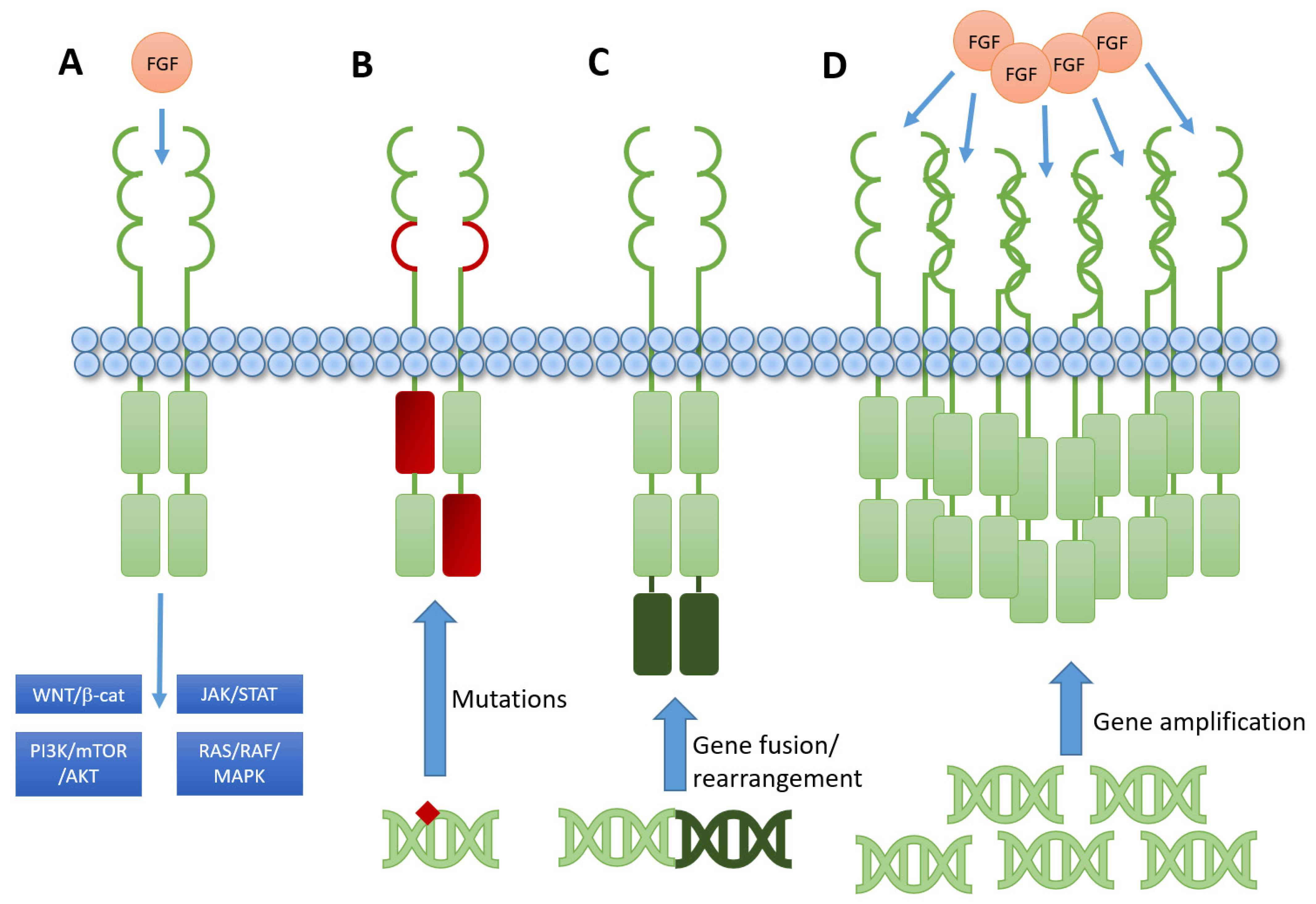
Cells Free Full Text Patient Selection Approaches In Fgfr Inhibitor The phase i ii fight 101 study (nct02393248) evaluated safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy of pemigatinib, a potent and selective fibroblast growth factor receptor (fgfr) 1 3 inhibitor, as monotherapy or in combination therapy, for refractory advanced malignancies, with and without fibroblast growth factor (fgf) and receptor (fgfr) gene alterations. Subbiah, v. et al. fight 101, a first in human study of potent and selective fgfr 1 3 inhibitor pemigatinib in pan cancer patients with fgf fgfr alterations and advanced malignancies. ann. oncol. Pemigatinib is an oral, potent, selective fibroblast growth factor receptor (fgfr) 1 3 inhibitor. fight 101, a three part, open label, first in human, phase i ii study (nct02393248), evaluated pemigatinib in patients with advanced solid tumors. in parts 1 and 2, pemigatinib monotherapy had a manageable safety profile and antitumor activity in fgfr altered tumors. part 3 (pemigatinib. Background: pemigatinib (incb054828) is a selective fibroblast growth factor receptor (fgfr) 1–3 inhibitor with demonstrated efficacy as monotherapy in phase 1 2 (fight 101) and phase 2 (fight 201, 202, 203) trials in pts with advanced cancer. here, we present preliminary safety, efficacy, and pharmacokinetic (pk) data for pemigatinib (pemi.

Targeted Therapies For Advanced Bladder Cancer New Strategies With Pemigatinib is an oral, potent, selective fibroblast growth factor receptor (fgfr) 1 3 inhibitor. fight 101, a three part, open label, first in human, phase i ii study (nct02393248), evaluated pemigatinib in patients with advanced solid tumors. in parts 1 and 2, pemigatinib monotherapy had a manageable safety profile and antitumor activity in fgfr altered tumors. part 3 (pemigatinib. Background: pemigatinib (incb054828) is a selective fibroblast growth factor receptor (fgfr) 1–3 inhibitor with demonstrated efficacy as monotherapy in phase 1 2 (fight 101) and phase 2 (fight 201, 202, 203) trials in pts with advanced cancer. here, we present preliminary safety, efficacy, and pharmacokinetic (pk) data for pemigatinib (pemi. Subbiah v, iannotti no, gutierrez m, smith dc, feliz l, lihou cf, tian c, silverman im, ji t, saleh m. fight 101, a first in human study of potent and selective fgfr 1 3 inhibitor pemigatinib in pan cancer patients with fgf fgfr alterations and advanced malignancies. ann oncol. 2022 may;33(5):522 533. doi: 10.1016 j.annonc.2022.02.001. epub 2022 feb 14. @article{subbiah2022fight101af, title={fight 101, a first in human study of potent and selective fgfr 1 3 inhibitor pemigatinib in pan cancer patients with fgf fgfr alterations and advanced malignancies.}, author={vivek subbiah and nicholas o iannotti and monica gutierrez and d. c. smith and luis f{\'e}liz and christine francis lihou and.
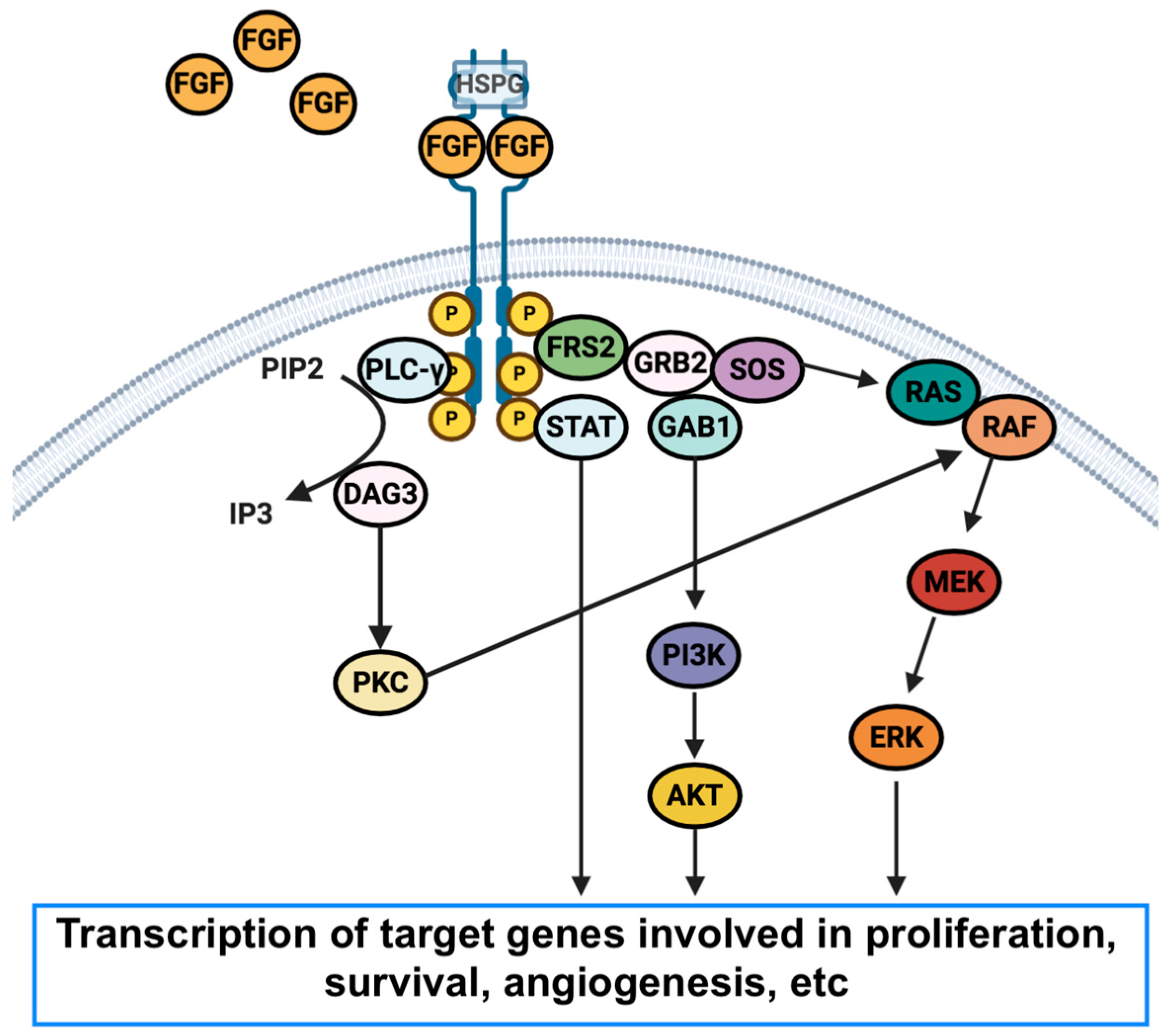
Medicina Free Full Text Targeted Therapies In Advanced Subbiah v, iannotti no, gutierrez m, smith dc, feliz l, lihou cf, tian c, silverman im, ji t, saleh m. fight 101, a first in human study of potent and selective fgfr 1 3 inhibitor pemigatinib in pan cancer patients with fgf fgfr alterations and advanced malignancies. ann oncol. 2022 may;33(5):522 533. doi: 10.1016 j.annonc.2022.02.001. epub 2022 feb 14. @article{subbiah2022fight101af, title={fight 101, a first in human study of potent and selective fgfr 1 3 inhibitor pemigatinib in pan cancer patients with fgf fgfr alterations and advanced malignancies.}, author={vivek subbiah and nicholas o iannotti and monica gutierrez and d. c. smith and luis f{\'e}liz and christine francis lihou and.

Comments are closed.