Diagram Catalytic Converter
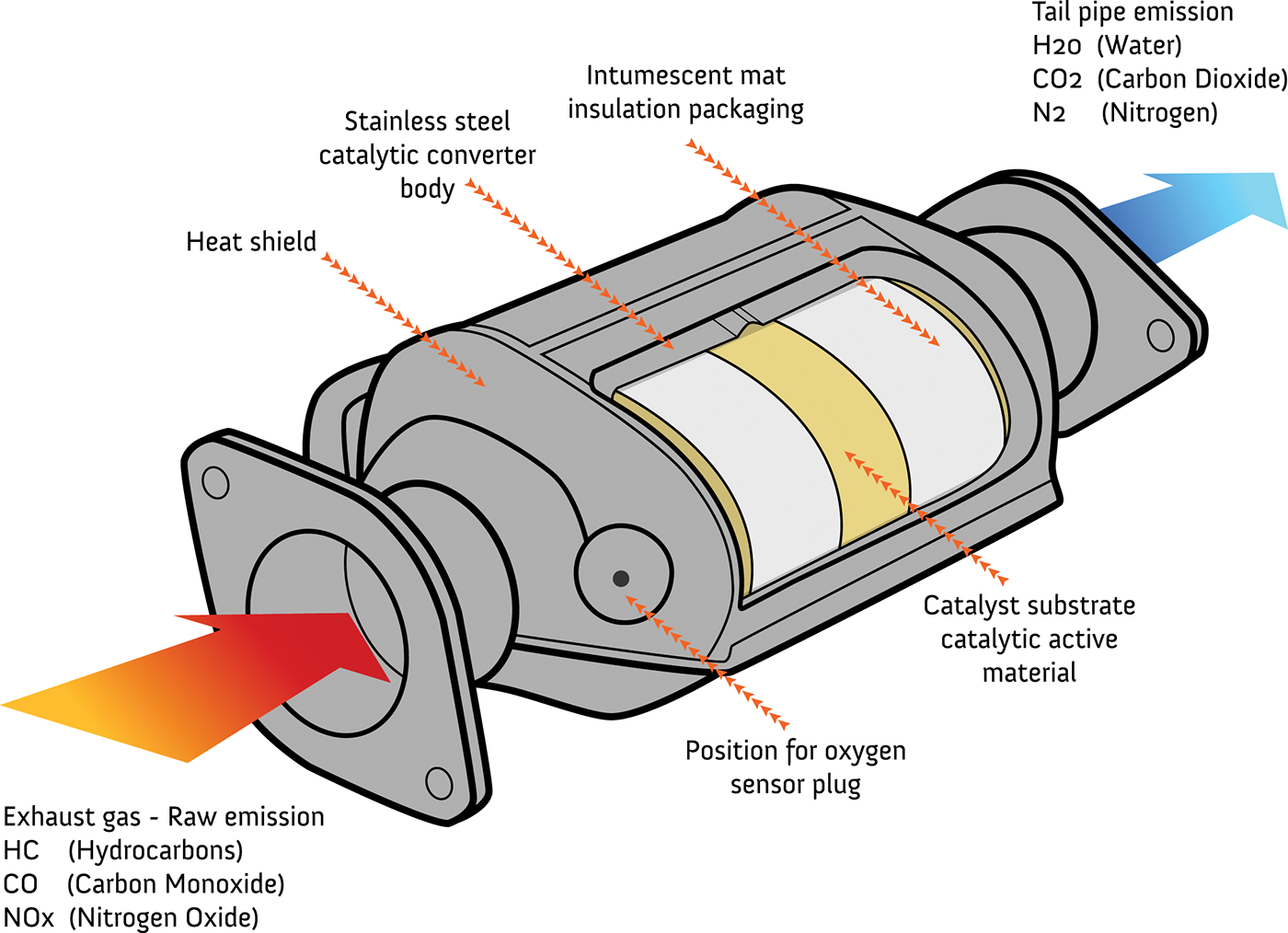
Catalytic Converters How They Work At Richard Santoro Blog A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. one of them (the converter's "input") is connected to the engine and brings in hot, polluted fumes from the engine's cylinders (where the fuel burns and produces power). A catalytic converter is an exhaust emission control device which converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less toxic pollutants by catalyzing a redox reaction. catalytic converters are usually used with internal combustion engines fueled by gasoline or diesel, including lean burn engines, and.

How A Catalytic Converter Works Napa Know How Blog A catalytic converter is an exhaust emission control device that uses a catalyst to convert three harmful compounds in car exhaust. monty rakusen getty images image source. there are millions of cars on the road in the united states, and each is a source of air pollution. in an attempt to stem this pollution, cities, states and the federal government create clean air laws that restrict the. A catalytic converter is a simple device that uses basic redox reactions to reduce the pollutants a car makes. it converts around 98% of the harmful fumes produced by a car engine into less harmful gases. it is composed of a metal housing with a ceramic honeycomb like interior with insulating layers. this honeycomb interior has thin wall. A catalytic converter is a device used in the exhaust system of internal combustion engines, primarily in vehicles, to reduce the harmful emissions produced during the combustion process. it works by facilitating chemical reactions that convert pollutants and harmful gases, such as nitrogen oxides (nox), carbon monoxide (co), and unburned. Step 2: substrate. inside the catalytic converter, you’ll find a substrate, which is a ceramic or metallic honeycomb like structure. the substrate is coated with precious metals such as platinum, palladium, and rhodium. these metals act as catalysts in the conversion of harmful gases into less harmful substances.
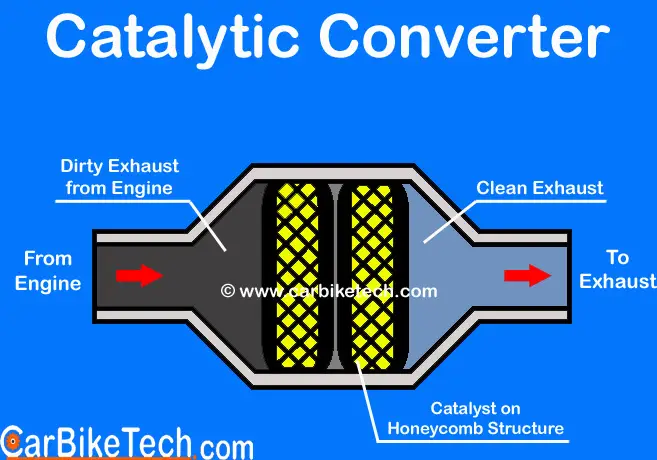
What Is A Catalytic Converter How Does It Work Carbiketech A catalytic converter is a device used in the exhaust system of internal combustion engines, primarily in vehicles, to reduce the harmful emissions produced during the combustion process. it works by facilitating chemical reactions that convert pollutants and harmful gases, such as nitrogen oxides (nox), carbon monoxide (co), and unburned. Step 2: substrate. inside the catalytic converter, you’ll find a substrate, which is a ceramic or metallic honeycomb like structure. the substrate is coated with precious metals such as platinum, palladium, and rhodium. these metals act as catalysts in the conversion of harmful gases into less harmful substances. In this video, you'll learn how a catalytic converter (cat) works.also check out our video on how a diesel particulate filter (dpf) works 👉 bit.ly 3. The average catalytic converter contains about 1 2 grams of rhodium, about 3 7 grams of platinum, and between 2 and 7 grams of palladium. while the market value of these metals has decreased, they.

Comments are closed.