Consider A Carnot Cycle Heat Pump Genlasopa
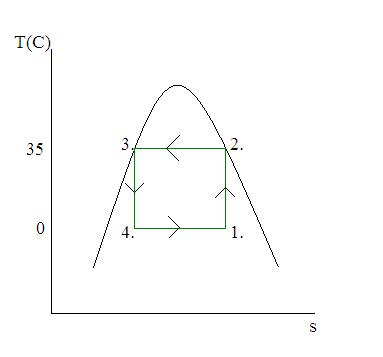
Consider A Carnot Cycle Heat Pump Genlasopa Solution. consider a carnot heat pump cycle executed in a steady flow system in the saturated liquid vapor mixture region using refrigerant 134a flowing at a rate of 0.195 kg s as the working fluid. it is known that the maximum absolute temperature in the cycle is 1.2 times the minimum absolute temperature, and the net power input to the cycle. Chapter 6: problem 135. consider a carnot heat pump cycle executed in a steady flow system in the saturated liquid vapor mixture region using refrigerant 134a flowing at a rate of 0.22 kg s as the working fluid. it is known that the maximum absolute temperature in the cycle is 1.2 times the minimum absolute temperature, and the net power.

Consider A Carnot Cycle Heat Pump Genlasopa A carnot heat pump (or carnot refrigerator) is a reverse carnot heat engine, that absorbs heat from a cold thermal reservoir and transfers it to a warmer thermal reservoir. as we will show below, it is the most efficient heat pump operating between two given temperatures. first, we will determine the coefficient of performance of the carnot. Here’s the best way to solve it. consider a carnot cycle heat pump with r 22 as the working fluid. heat is rejected from the r 22 at 100 f. during which process the r 22 changes from saturated vapor to saturated liquid. the heat is transferred to the r 22 at 30 f. show the cycle on a t s diagram. find the quality of the r 22 at the beginning. Chapter 8: problem 34. consider a carnot cycle heat pump with r − 22 as the working fluid. heat is rejected from the r − 22 at 40 ∘ c, during which process the r − 22 changes from saturated vapor to saturated liquid. the heat is transferred to the r − 22 at 0 ∘ c. a. show the cycle on a t − s diagram. b. A carnot heat pump imagine a carnot heat pump operates between an outside temperature of 0 °c 0 °c and an inside temperature of 20.0 °c 20.0 °c. what is the work needed if the heat delivered to the inside of the house is 30.0 kj? strategy because the heat pump is assumed to be a carnot pump, its performance coefficient is given by k p = q h.
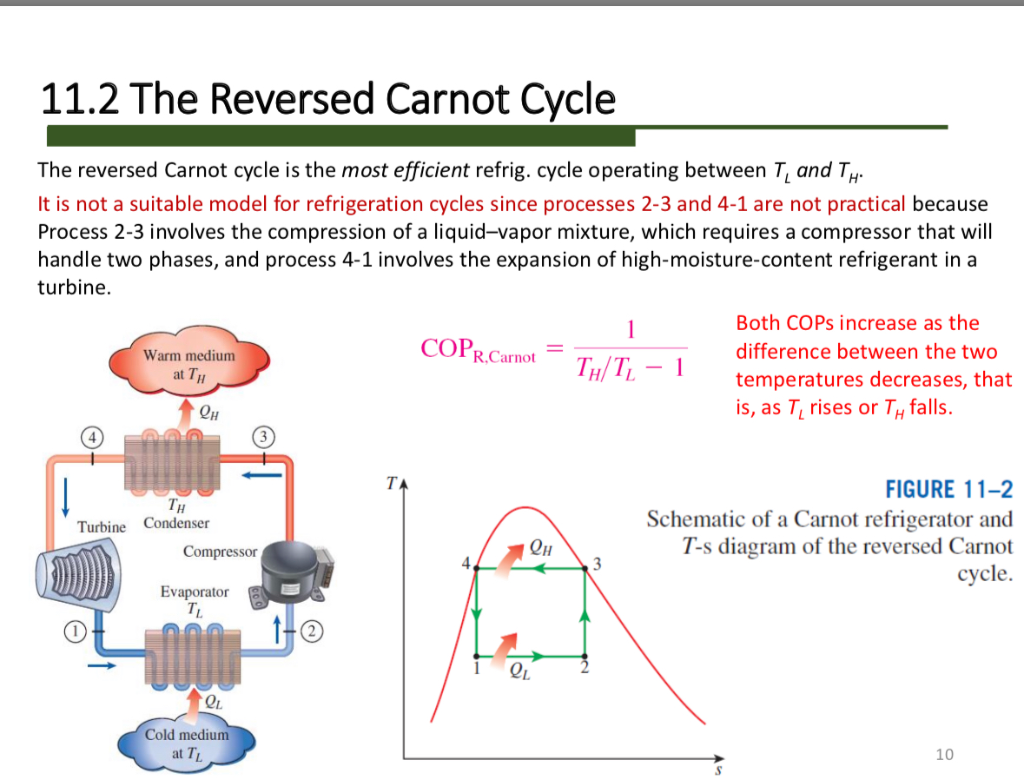
Carnot Cycle Heat Pump Lasopacute Chapter 8: problem 34. consider a carnot cycle heat pump with r − 22 as the working fluid. heat is rejected from the r − 22 at 40 ∘ c, during which process the r − 22 changes from saturated vapor to saturated liquid. the heat is transferred to the r − 22 at 0 ∘ c. a. show the cycle on a t − s diagram. b. A carnot heat pump imagine a carnot heat pump operates between an outside temperature of 0 °c 0 °c and an inside temperature of 20.0 °c 20.0 °c. what is the work needed if the heat delivered to the inside of the house is 30.0 kj? strategy because the heat pump is assumed to be a carnot pump, its performance coefficient is given by k p = q h. The basic components of a heat pump in its heating mode are shown in figure 15.5.3. a working fluid such as a non cfc refrigerant is used. in the outdoor coils (the evaporator), heat transfer qc occurs to the working fluid from the cold outdoor air, turning it into a gas. 3. the work done on the gas in one cycle of the carnot refrigerator is shown and given by the area enclosed by the loop mponm. the work done on the ideal gas is equal to the area enclosed by the path of the pv diagram. from the first law, this work is given by. w = qh −qc. (4.6.13) (4.6.13) w = q h − q c.

Carnot Cycle Heat Pump Grlasopa The basic components of a heat pump in its heating mode are shown in figure 15.5.3. a working fluid such as a non cfc refrigerant is used. in the outdoor coils (the evaporator), heat transfer qc occurs to the working fluid from the cold outdoor air, turning it into a gas. 3. the work done on the gas in one cycle of the carnot refrigerator is shown and given by the area enclosed by the loop mponm. the work done on the ideal gas is equal to the area enclosed by the path of the pv diagram. from the first law, this work is given by. w = qh −qc. (4.6.13) (4.6.13) w = q h − q c.

A Carnot Heat Pump Cycle Operating At Steady State With Ammonia As The

Comments are closed.