Comparison Of Work And Heat

Comparison Of Work And Heat Youtube The maximum amount of work one can attain from heat is given by the carnot efficiency. heat is the energy associated with the random motion of particles, while work is the energy of ordered motion in one direction. therefore heat is "low quality" energy and work is "high quality" energy, and this supports the entropy statement of the second law. While both involve energy transfer, they differ in their mechanisms, effects, quantification, and conversion. heat is the transfer of energy due to a temperature difference, while work is the transfer of energy due to the application of a force. understanding the attributes of heat and work is essential in comprehending the behavior of physical.
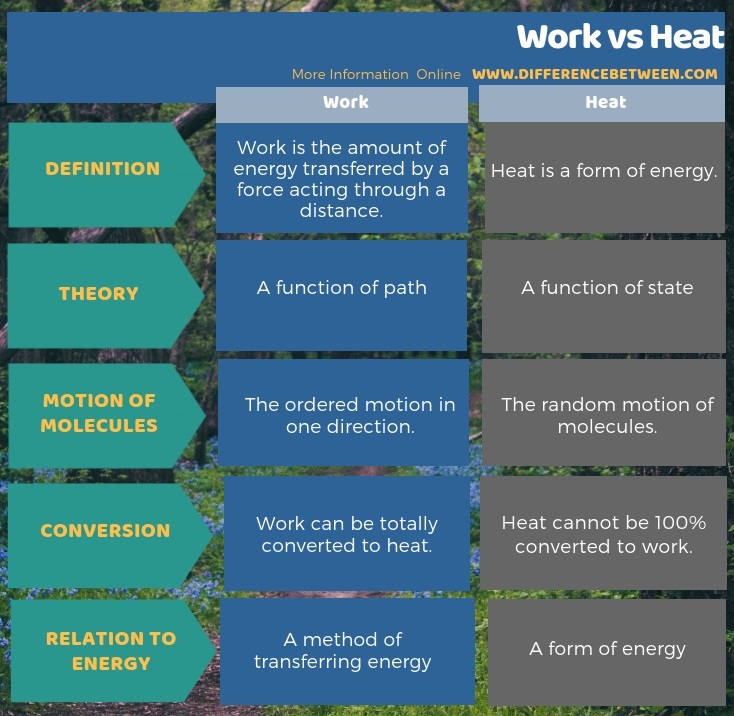
Difference Between Work And Heat Compare The Difference Between Solution. first we need to determine the change in volume, Δ v. a change is always the final value minus the initial value: Δ v = v final − v initial = 6.19 l − 3.44 l = 2.75 l. now we can use the definition of work to determine the work done: w = − p ext · Δ v = − (1.26 atm) (2.75 l) = −3.47 l·atm. In thermodynamics the meaning of heat is more precise: it is a process, a way energy can move. heat is energy that moves from a hot object to a cold object. when heat leaves the system, it has a negative sign, and when it enters the system, it has a positive sign. heat and work are the ways that energy can move between objects. Energy, heat and work. energy is the ability to move an object against a resisting force. moving an object against a resisting force is called work, so we can write our energy definition more formally: energy is the ability to do work. work is a way to transfer energy from one collection of matter to another. This page titled 3.3: work, heat, and internal energy is shared under a cc by 4.0 license and was authored, remixed, and or curated by openstax via source content that was edited to the style and standards of the libretexts platform. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure.

Difference Between Heat And Work Heat And Work Difference Youtube Energy, heat and work. energy is the ability to move an object against a resisting force. moving an object against a resisting force is called work, so we can write our energy definition more formally: energy is the ability to do work. work is a way to transfer energy from one collection of matter to another. This page titled 3.3: work, heat, and internal energy is shared under a cc by 4.0 license and was authored, remixed, and or curated by openstax via source content that was edited to the style and standards of the libretexts platform. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. Work cannot be converted 100% into heat. form. heat is a low quality energy. work is a high quality energy. transfer. temperature difference is the driving force for heat transfer. displacement or change in position is behind the work transfer. nature. heat is associated with the random motion of particles. Summary. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. heat is the energy transferred between two objects (or two parts of a system) because of a temperature difference. internal energy of a thermodynamic system is its total mechanical energy.

Ppt Chapter 17 Powerpoint Presentation Free Download Id 1792465 Work cannot be converted 100% into heat. form. heat is a low quality energy. work is a high quality energy. transfer. temperature difference is the driving force for heat transfer. displacement or change in position is behind the work transfer. nature. heat is associated with the random motion of particles. Summary. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. heat is the energy transferred between two objects (or two parts of a system) because of a temperature difference. internal energy of a thermodynamic system is its total mechanical energy.

Chapter 17 Free Energy And Thermodynamics Ppt Download

Work Vs Heat What S The Difference

Comments are closed.