Chem 1411 Chapter 2 Part 3 Average Atomic Mass

Chem 1411 Chapter 2 Part 3 Average Atomic Mass Youtube About press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket press copyright. Empirical molar mass = 14.01 g 2(16.00 g) = 46.01 g. next, we determine the ratio between the molar mass and the empirical molar mass. the molar mass is twice the empirical molar mass. this means that there are two no2 units in each molecule of the compound, and the molecular formula is (no2)2 or n2o4.

Calculating Atomic Mass Isotopes And Abundance Course Hero Chemistry 1411 chapter 2. john dalton***. in the early 1800's, john dalton (he began teaching at age 12) proposed an atomic model based on facts and experimental evidence. his theory can summed up as follows: all matter is composed of very tiny indivisible particles called atoms. Chem 1411 practice exam i (chapters 1, 2, 3): 25 questions. q1‐7: chapter 1; q8‐13: chapter 2; q14 note that the average atomic mass (with decimal) can be. Chem 1411 chapter 1. 25 terms. aaspano04. med chem exam 1 part 3. 102 terms. sosburn23. what isotopes have a greater effect on the average atomic mass?. Chemistry report draft 2; db2 bc fxhcgjvgkjlk; practice packet, reactions, 2022 key; chem 1411 chapter 3 notes part 1: chemical equations, reaction types and predictability, chapter 3 notes part 2 formula weight, molecular weight, moles, percent composition.

Chapter 2 Elements Ions And Molecules Chem 1411 Studocu Chem 1411 chapter 1. 25 terms. aaspano04. med chem exam 1 part 3. 102 terms. sosburn23. what isotopes have a greater effect on the average atomic mass?. Chemistry report draft 2; db2 bc fxhcgjvgkjlk; practice packet, reactions, 2022 key; chem 1411 chapter 3 notes part 1: chemical equations, reaction types and predictability, chapter 3 notes part 2 formula weight, molecular weight, moles, percent composition. The average atomic mass for an element can be easily calculated by taking the average of the atomic mass units of each isotope. atomic mass of copper is 63.54 amu. there are 3 naturally occurring isotopes of hydrogen and 2 naturally occurring isotopes of copper. calculations of atomic mass use the percent abundance of each isotope. In one step: (0.7577 × 34.969) (0.2423 × 36.966) = 35.46amu (0.7577 × 34.969) (0.2423 × 36.966) = 35.46 amu. step 3: think about your result. the calculated average atomic mass is closer to 35 than to 37 because a greater percentage of naturally occurring chlorine atoms have the mass number of 35. it agrees with the value from the table.

Chem 1411 Ch 9 Notes Ch 9 Reactions In Aqueous Solutions Molarity The average atomic mass for an element can be easily calculated by taking the average of the atomic mass units of each isotope. atomic mass of copper is 63.54 amu. there are 3 naturally occurring isotopes of hydrogen and 2 naturally occurring isotopes of copper. calculations of atomic mass use the percent abundance of each isotope. In one step: (0.7577 × 34.969) (0.2423 × 36.966) = 35.46amu (0.7577 × 34.969) (0.2423 × 36.966) = 35.46 amu. step 3: think about your result. the calculated average atomic mass is closer to 35 than to 37 because a greater percentage of naturally occurring chlorine atoms have the mass number of 35. it agrees with the value from the table.
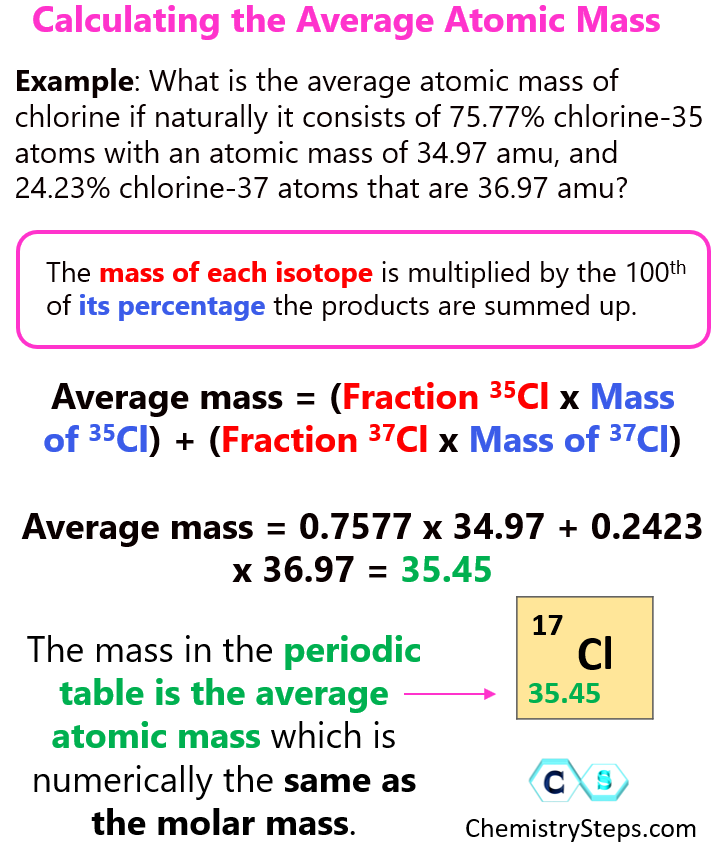
How To Calculate The Average Atomic Mass Chemistry Steps

Comments are closed.