Catalytic Converters How They Work At Richard Santoro Blog
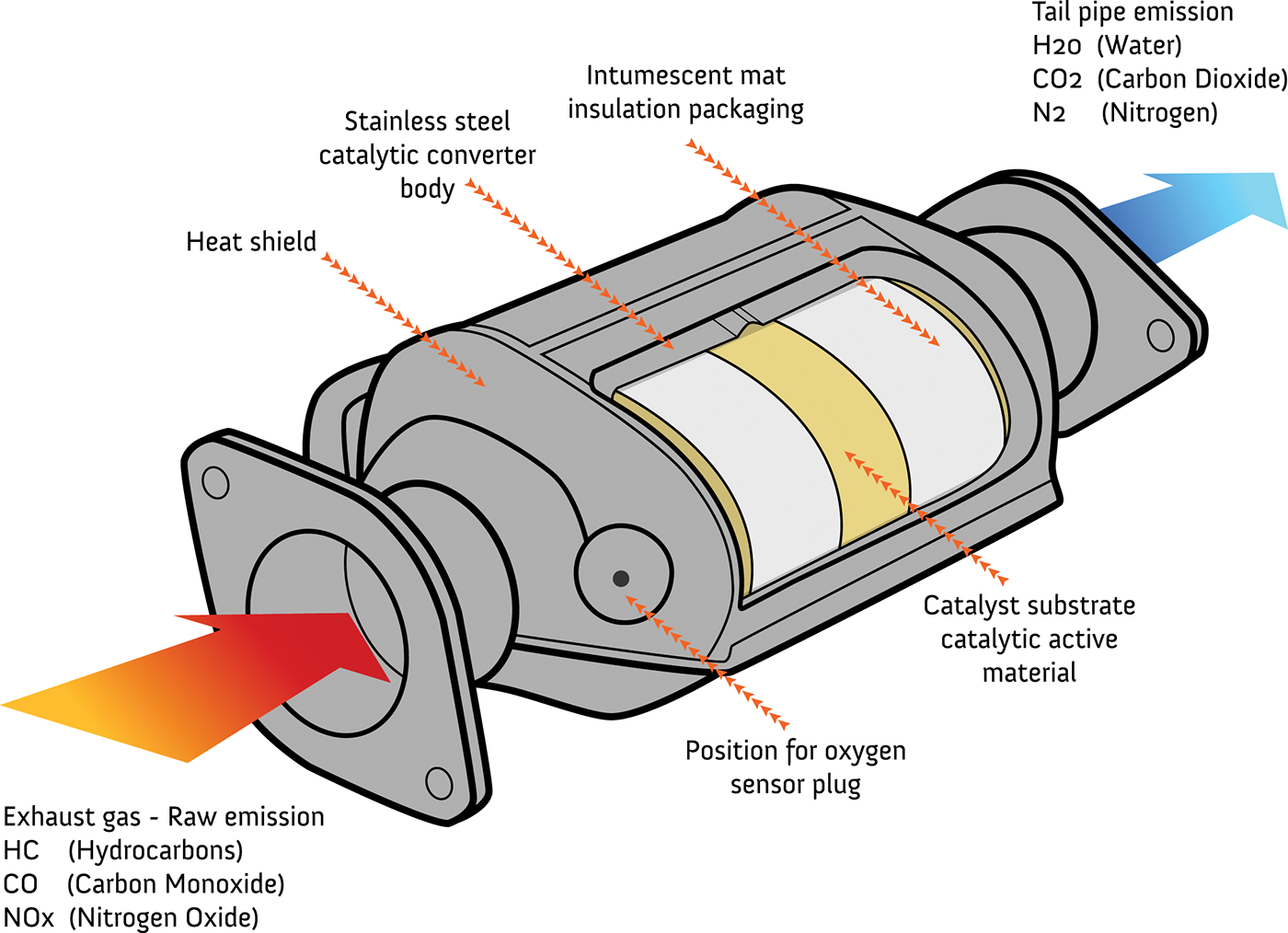
Catalytic Converters How They Work At Richard Santoro Blog In a catalytic converter, the catalyst's job is to speed up the removal of pollution. the catalyst is made from platinum or a similar, platinum like metal such as palladium or rhodium. a catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. Overview of the catalytic converter. the catalytic converter, often referred to as the "cat" or "cat converter," is a device designed to control and convert harmful exhaust emissions into less harmful compounds. it acts as an environmental warrior, curbing the release of pollutants and mitigating their impact on our planet.
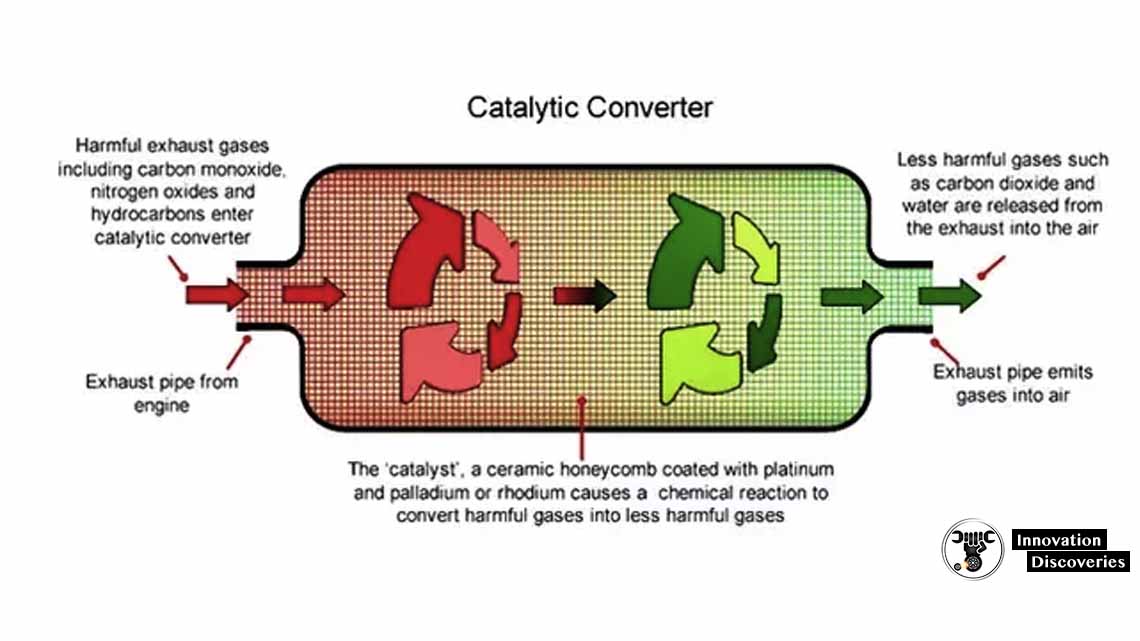
Catalytic Converters How They Work At Richard Santoro Blog How does a catalytic converter work? catalytic converters use chemical reactions to transform harmful gases into less harmful ones. these reactions occur on the surface of a catalyst material inside the converter. the converter typically contains two types of catalysts: a reduction catalyst and an oxidation catalyst. 1. reduction catalyst:. When an no or no 2 molecule contacts the catalyst, the catalyst rips the nitrogen atom out of the molecule and holds on to it, freeing the oxygen in the form of o 2. the nitrogen atoms bond with other nitrogen atoms that are also stuck to the catalyst, forming n 2. for example: 2no => n 2 o 2 or 2no 2 => n 2 2o 2. A catalytic converter works similarly to a water filter: once impure water passes through a water filter, it comes out as clean water that is safe for drinking. at its core, the converter uses a catalyst—comprising precious metals like platinum, palladium, and rhodium—to accelerate the chemical reactions that convert harmful gases into. Catalytic converters – the basics. catalytic converters are devices that use a catalyst in order to convert harmful compounds found in car exhaust into less harmful alternatives. the three compounds that are converted by the catalytic converter are: nitrogen oxides: these are created when the engine’s heat forces nitrogen in the air to mix.

Catalytic Converters How They Work At Richard Santoro Blog A catalytic converter works similarly to a water filter: once impure water passes through a water filter, it comes out as clean water that is safe for drinking. at its core, the converter uses a catalyst—comprising precious metals like platinum, palladium, and rhodium—to accelerate the chemical reactions that convert harmful gases into. Catalytic converters – the basics. catalytic converters are devices that use a catalyst in order to convert harmful compounds found in car exhaust into less harmful alternatives. the three compounds that are converted by the catalytic converter are: nitrogen oxides: these are created when the engine’s heat forces nitrogen in the air to mix. Catalytic converters rely heavily on the heat of the exhaust to work. when cold, they provide almost zero benefits, which is why modern cars have a cold start program that sounds and feels. Two way catalytic converters: these are like basic cleaners. they work mainly in diesel engines, turning two bad things in the exhaust (carbon monoxide and unburned fuel bits) into less harmful stuff like carbon dioxide and water. three way catalytic converters: this is what most regular cars use. it's like a three in one cleaner, getting rid.

Catalytic Converters How They Work At Richard Santoro Blog Catalytic converters rely heavily on the heat of the exhaust to work. when cold, they provide almost zero benefits, which is why modern cars have a cold start program that sounds and feels. Two way catalytic converters: these are like basic cleaners. they work mainly in diesel engines, turning two bad things in the exhaust (carbon monoxide and unburned fuel bits) into less harmful stuff like carbon dioxide and water. three way catalytic converters: this is what most regular cars use. it's like a three in one cleaner, getting rid.
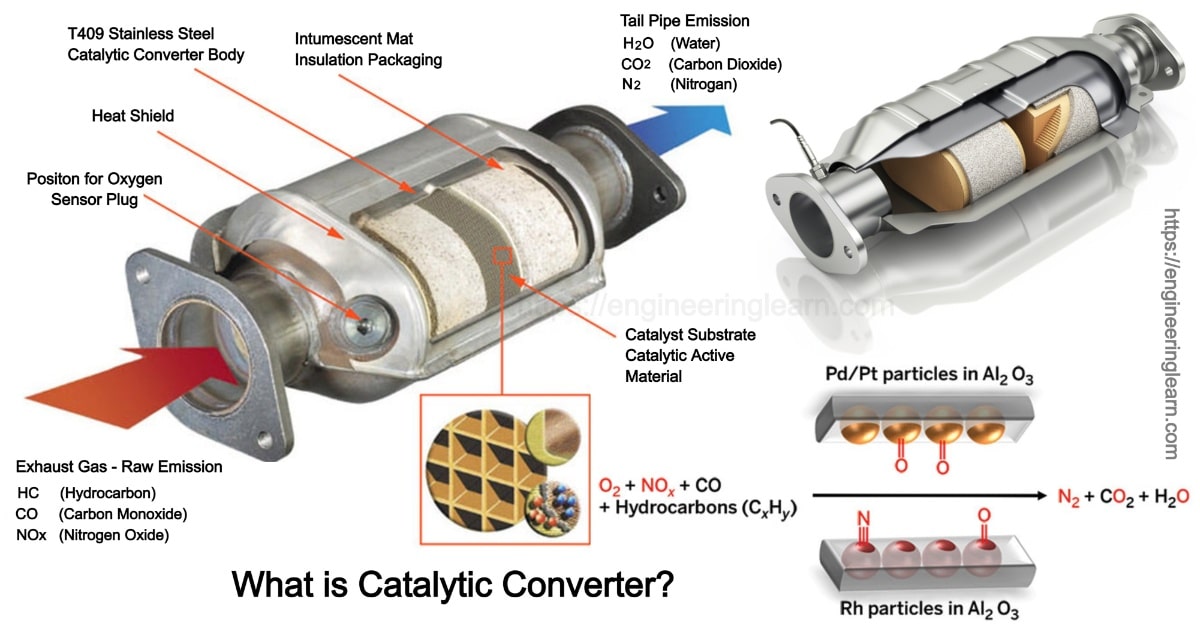
Catalytic Converters How They Work At Richard Santoro Blog

Comments are closed.