C3h6o Isomers Functional Structural Aliphatic Cyclic
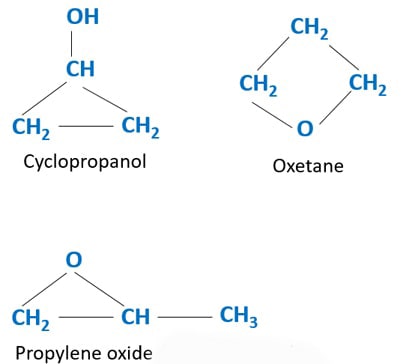
C3h6o Isomers Functional Structural Aliphatic Cyclic 6. o isomers | functional, structural, aliphatic, cyclic. for c 3 h 6 o chemical formula, we can draw different isomers. c 3 h 6 o is a chemical formula for several organic compounds. therefore, there should be different isomers. aldehyde compounds, ketone compounds, cyclic alcohol compounds and more isomers can be drawn for c 3 h 6 o. I count 11 isomers of "c" 3"h" 6"o". > they are: prop 2 en 1 ol (from commons.wikimedia.org) methoxyethene propanone (from meritnation ) and its tautomer, prop 1 en 2 ol propanal (from commons.wikimedia.org) and its two tautomeric isomers (z) prop 1 en 1 ol and (e) prop 1 en 1 ol then we have cyclopropanol oxetane (r) methyloxirane (s) methyloxirane and that makes 11 isomers!.

Isomers Of C3h6o Functional group isomerism. in this variety of structural isomerism, the isomers contain different functional groups that is, they belong to different families of compounds (different homologous series). example 3: isomers in c 3 h 6 o. a molecular formula c3h6o c 3 h 6 o could be either propanal (an aldehyde) or propanone (a ketone). A molecular formula. c3h6o (3.4.7) (3.4.7) c 3 h 6 o. could be either propanal (an aldehyde) or propanone (a ketone). there are other possibilities as well for this same molecular formula – for example, you could have a carbon carbon double bond (an alkene) and an oh group (an alcohol) in the same molecule. The cis isomer has the two single hydrogen atoms on the same side of the molecule, while the trans isomer has them on opposite sides of the molecule. in both molecules, the bonding order of the atoms is the same. in order for geometric isomers to exist, there must be a rigid structure in the molecule to prevent free rotation around a bond. Position isomerism. in position isomerism, the basic carbon skeleton remains unchanged, but important groups are moved around on that skeleton. for example, there are two structural isomers with the molecular formula c 3 h 7 br. in one of them the bromine atom is on the end of the chain, whereas in the other it's attached in the middle.

C3h6o Isomers Functional Structural Aliphatic Cyclic The cis isomer has the two single hydrogen atoms on the same side of the molecule, while the trans isomer has them on opposite sides of the molecule. in both molecules, the bonding order of the atoms is the same. in order for geometric isomers to exist, there must be a rigid structure in the molecule to prevent free rotation around a bond. Position isomerism. in position isomerism, the basic carbon skeleton remains unchanged, but important groups are moved around on that skeleton. for example, there are two structural isomers with the molecular formula c 3 h 7 br. in one of them the bromine atom is on the end of the chain, whereas in the other it's attached in the middle. For the molecular formula c3h6o draw structural isomers with the following functional groups. 1. alkene and alcohol 2. cyclic ether 3. ketone 4. aldehyde 5. cyclic alcohol 6. epoxide 7. alkene and ether. for the molecular formula c4h9no draw structural isomers with the following functional groups. Constitutional isomers. iupac defines constitutional isomerism as “isomerism between structures differing in constitution and described by different line formulae e.g. ch3och3 and ch3ch2oh.”. recall that there are three types of constitutional isomer commonly seen: chain, positional and functional.

Isomers Of C3h6o For the molecular formula c3h6o draw structural isomers with the following functional groups. 1. alkene and alcohol 2. cyclic ether 3. ketone 4. aldehyde 5. cyclic alcohol 6. epoxide 7. alkene and ether. for the molecular formula c4h9no draw structural isomers with the following functional groups. Constitutional isomers. iupac defines constitutional isomerism as “isomerism between structures differing in constitution and described by different line formulae e.g. ch3och3 and ch3ch2oh.”. recall that there are three types of constitutional isomer commonly seen: chain, positional and functional.
Two Isomers Of C3h6o

Comments are closed.