Ap Chem Unit 7 Equilibrium Practice Problems 4 A

Ap Chem Unit 7 Equilibrium Practice Problems 4 A Youtube Chemical equilibrium involves the balance between the forward and reverse reactions in a closed system (n2 3h2 ⇌ 2nh3) physical equilibrium occurs when a substance can exist in more than one physical state (solid, liquid, or gas) and the rates of interconversion between these states are equal. vapor liquid equilibrium (h2o (l) ⇌ h2o (g)). Equilibrium problems advanced placement level. return to equilibrium menu. problem #1: when 0.0322 mol of no and 1.70 g of bromine are placed in a 1.00 l reaction vessel and sealed, the mixture reacts and the following equilibrium is established: 2no (g) br 2 (g) ⇌ 2nobr (g) at 25.0 °c the equilibrium of nitrosyl bromide is 0.438 atm.

Ap Chemistry Unit 7 Test Equilibrium By Science Short Stop Tpt A. since q= [cl2] [co] [cocl2], when cl2 (g) is added to the system q>kq>k and the system will restore equilibrium by producing more cocl2 (g) for which of the following salts would the relationship between molar solubility, s, in mol l, and the value of ksp be represented by the equation ksp=4s3. c. Forward reaction rate is faster than the reverse and concentration is equal. what is the correct equilibrium expression for the following reaction: 2h o (aq) ↔ 2h o (l) o (g) when 0.40 mole of so and 0.60 mole of o are placed in an evacuated 1.00–liter flask, the reaction represented below occurs. after the reactants and the product reach. Suppose a chemist adds 1 mole of pure ab (g) to a sealed vessel. a) describe the process of achieving equilibrium in terms of the changing of concentrations of ab (g), a (g), and b (g) • we start out with only ab gas in a vessel. • as time goes on, [ab] drops. • at the same time, [a] and [b] r increasing. Ap chemistry unit 7 homework problems equilibrium and k sp nature of the equilibrium state 1. draw on this graph where equilibrium has been reached. 2. what are three qualities of any equilibrium equation? a. reversible b. dynamic c. occur when there are a stable ratio of products: reactants 3.
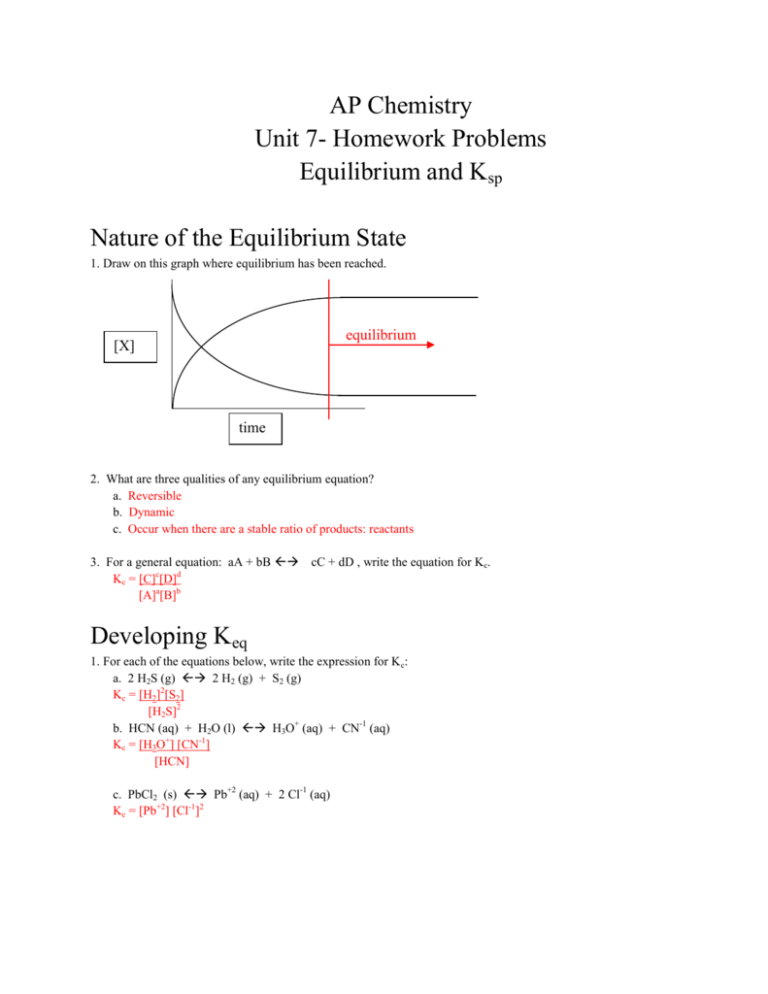
Ap Chemistry Unit 7 Homework Problems Equilibrium And Ksp Suppose a chemist adds 1 mole of pure ab (g) to a sealed vessel. a) describe the process of achieving equilibrium in terms of the changing of concentrations of ab (g), a (g), and b (g) • we start out with only ab gas in a vessel. • as time goes on, [ab] drops. • at the same time, [a] and [b] r increasing. Ap chemistry unit 7 homework problems equilibrium and k sp nature of the equilibrium state 1. draw on this graph where equilibrium has been reached. 2. what are three qualities of any equilibrium equation? a. reversible b. dynamic c. occur when there are a stable ratio of products: reactants 3. In this video, mr. krug shows students how to solve equilibrium problems using the ice (or rice) box method, using the information you know in a problem to s. 66 multiple choice questions. definition. at equilibrium, . all chemical reactions have ceased. ·. the rates of the forward and reverse reactions are equal the rate constants of the forward and reverse reactions are equal. the value of the equilibrium constant is 1. the limiting reagent has been consumed.

Ap Chem Unit 7 Equilibrium Practice Problems 1 Youtube In this video, mr. krug shows students how to solve equilibrium problems using the ice (or rice) box method, using the information you know in a problem to s. 66 multiple choice questions. definition. at equilibrium, . all chemical reactions have ceased. ·. the rates of the forward and reverse reactions are equal the rate constants of the forward and reverse reactions are equal. the value of the equilibrium constant is 1. the limiting reagent has been consumed.

Comments are closed.