Answered Iupac Nomenclature Of Alkanes And Bartleby

Answered Applying The Iupac Nomenclature System Bartleby Iupac nomenclature of alkanes and cycloalkanes provide the iupac name for each of the following structures, following this manner as shown 4 sthyl 2,2 dimethufexane compound 5 compound 6 ch3 ch3 ch2 ch2 ch3 ch2 c ch2 ch2 ch3 ch3 3. ch2 ch2 c ch2 ch2ch2 ch3 ch2 Čh3 ch3. Give the proper iupac names of the following compounds. answer. a) this alkane has a 9 carbon longest continuous chain length that we number from left to right (structure on left numbered in blue) to make the ethyl substituent be number 4. for the structure on the right (numbered in red) going from right to left, the methyl substituent is number 4.

Answered 1 Name The Alkane Using Iupac Bartleby Now, let’s add another methyl group next to the first one: again, you have two options for numbering the parent chain. one gives the 3,4 and the other one 2,3 locants for the two methyl groups and 2,3 clearly beats 3,4. therefore, the final name of our compound is going to be 2,3 di methylpentane. Name the eight 5 carbon alkyl groups you drew in exercise 3.4.1. answer. pentyl, 1 methylbutyl, 1 ethylpropyl, 2 methylbutyl, 3 methylbutyl, 1,1 dimethylpropyl, 1,2 dimethylpropyl, 2,2 dimethylpropyl. exercise 3.4.4 3.4. 4. give the iupac name for the following hydrocarbon, and convert the drawing into a skeletal structure. answer. Alkanes are saturated organic compounds which are made up of c and h atoms. alkanes have the general formula of cnh2n 2. alkane contains sp3 hybridized carbon atoms with four sigmas (σ) bonds & every hydrogen atom is connected with one of the carbon atoms. the size of the alkane could be determined by the number of carbon atoms located in it. Iupac nomenclature of alkanes. identify the longest continuous carbon chain as the parent chain. this chain determines the parent name (or last name) of the alkane. if there are two choices of the same length, then the parent chain is the longest chain with the greatest number of “branches”. the term substituent will be used from now on as.
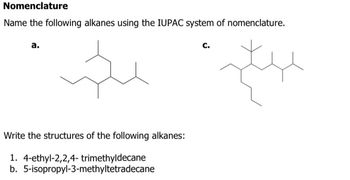
Answered Nomenclature Name The Following Alkanes Bartleby Alkanes are saturated organic compounds which are made up of c and h atoms. alkanes have the general formula of cnh2n 2. alkane contains sp3 hybridized carbon atoms with four sigmas (σ) bonds & every hydrogen atom is connected with one of the carbon atoms. the size of the alkane could be determined by the number of carbon atoms located in it. Iupac nomenclature of alkanes. identify the longest continuous carbon chain as the parent chain. this chain determines the parent name (or last name) of the alkane. if there are two choices of the same length, then the parent chain is the longest chain with the greatest number of “branches”. the term substituent will be used from now on as. Examples of alkane iupac naming. ch 4 methane. ch 3 ch 3 ethane. ch 3 ch 2 ch 3 propane. you can see every name in above example are finished with ane suffix. in the iupac nomenclature, names of alkane should be finished with ane suffix. also you see, another suffix (meth, eth, prop) is used in front of ane suffix. Answers the nomenclature of alkyl halides. give a systematic name for each of the following compounds: answers the nomenclature of alkenes. name the following alkenes according to the iupac nomenclature rules. determine if the configuration of the double bond is e or z and include it in the name: answers the nomenclature of alkynes.
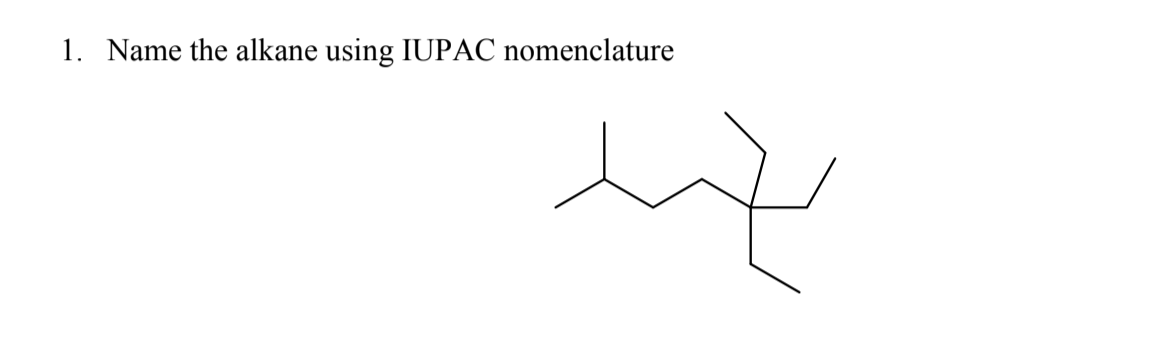
Answered 1 Name The Alkane Using Iupac Bartleby Examples of alkane iupac naming. ch 4 methane. ch 3 ch 3 ethane. ch 3 ch 2 ch 3 propane. you can see every name in above example are finished with ane suffix. in the iupac nomenclature, names of alkane should be finished with ane suffix. also you see, another suffix (meth, eth, prop) is used in front of ane suffix. Answers the nomenclature of alkyl halides. give a systematic name for each of the following compounds: answers the nomenclature of alkenes. name the following alkenes according to the iupac nomenclature rules. determine if the configuration of the double bond is e or z and include it in the name: answers the nomenclature of alkynes.
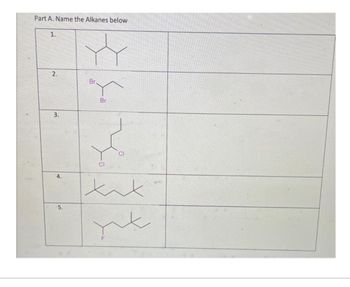
Answered Part A Name The Alkanes Below 1 2 3 Bartleby

Answered Iupac Nomenclature Of Alkanes And Bartleby

Comments are closed.