A Phase I Ii Trial Of Pirtobrutinib In Patients With R R Waldenstrom S
A Phase I Ii Trial Of Pirtobrutinib In Patients With R R Waldenstrom S The bruin study is a multicenter, international, phase i ii clinical trial designed to evaluate the safety and efficacy of pirtobrutinib in patients with r r b cell malignancies, reporting partial results in 2021. 6 in the phase i portion, patients received oral pirtobrutinib monotherapy in increasing doses to determine the recommended phase ii dose (rp2d) of 200 mg once daily given in. A single arm, phase ii study (nct02180724) of acalabrutinib reported a 24 month pfs rate of 90% for tn patients and 82% for r r patients. at a median duration of follow up of 27.4 months, orrs.

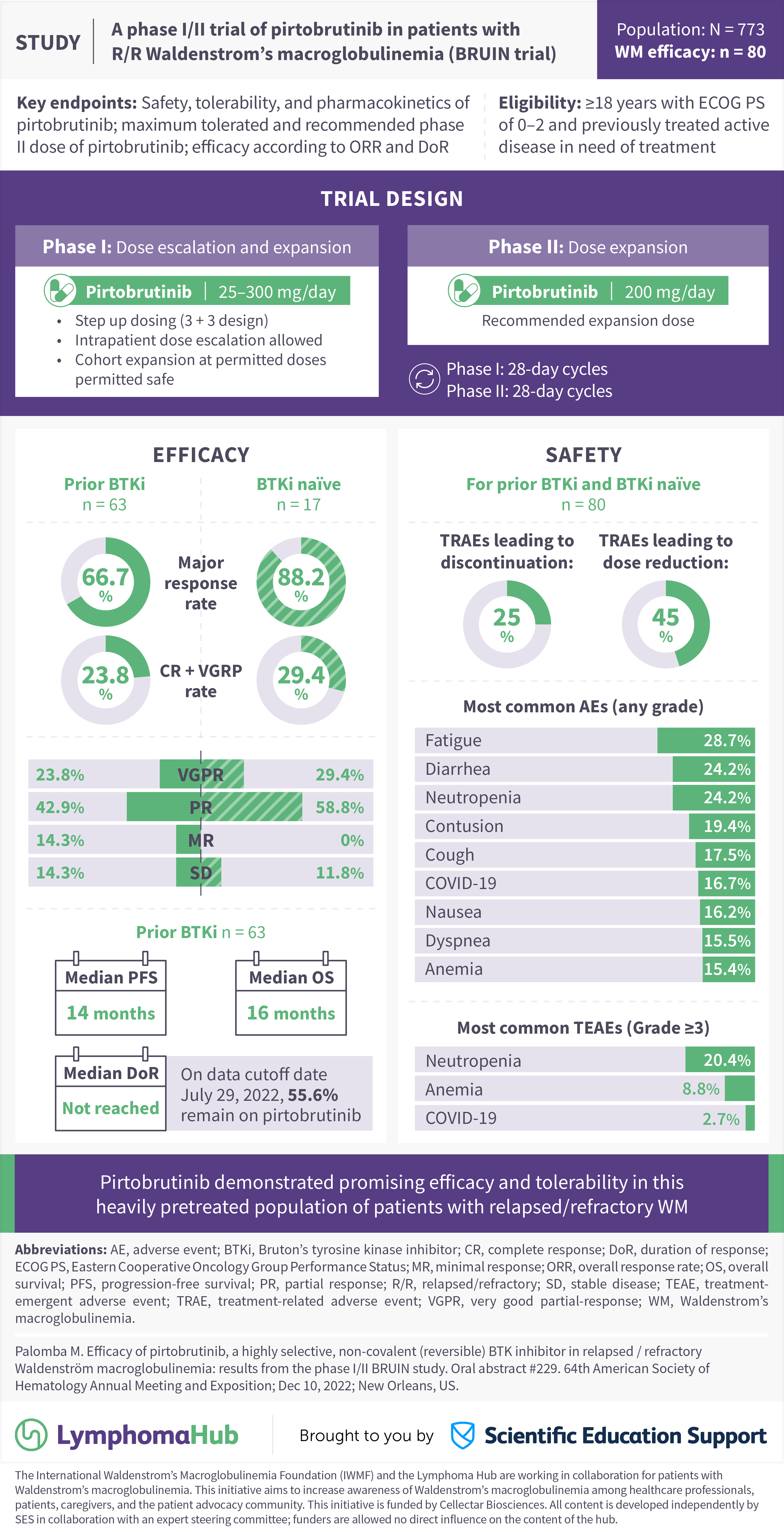
A Phase I Ii Trial Of Pirtobrutinib In Patients With R R Waldenstrom S The lymphoma hub is happy to present a visual abstract representing key data from the phase i ii bruin trial (nct03740529) evaluating the safety and efficacy of pirtobrutinib, a reversible (non covalent) bruton’s tyrosine kinase inhibitor, in heavily pretreated patients with relapsed refractory waldenstrom’s macroglobulinemia, as presented. Pirtobrutinib (formerly known as loxo 305) was reported to have good efficacy in the phase i ii bruin study (nct03740529) in patients with r r b cell malignancies. an orr of 68% was reported for 19 patients with r r wm, with partial responses in 50% of patients (no crs or vgprs). In the phase 1 2 bruin trial, pirtobrutinib was given alone in 323 patients with r r b cell malignancies, including 26 wm patients . in this population, the median number of prior therapies was 3 (range 2–4) and 18 (69%) had previously been treated with a covalent btki and discontinued due to progression ( n = 12) or toxicity or for another. The response evaluable cohort consisted of all relapsed refractory (r r) wm pts enrolled to either phase 1 or 2 who had undergone their first response assessment or discontinued therapy. the safety cohort consisted of all pts with b cell malignancies who received at least one dose of pirtobrutinib monotherapy (n=725).

Pirtobrutinib Venetoclax For Waldenström Macroglobulinemia Clinical In the phase 1 2 bruin trial, pirtobrutinib was given alone in 323 patients with r r b cell malignancies, including 26 wm patients . in this population, the median number of prior therapies was 3 (range 2–4) and 18 (69%) had previously been treated with a covalent btki and discontinued due to progression ( n = 12) or toxicity or for another. The response evaluable cohort consisted of all relapsed refractory (r r) wm pts enrolled to either phase 1 or 2 who had undergone their first response assessment or discontinued therapy. the safety cohort consisted of all pts with b cell malignancies who received at least one dose of pirtobrutinib monotherapy (n=725). Pirtobrutinib has been examined in the bruin study, a phase i ii, multicenter trial (nct03740529) assessing patients with r r b cell malignancies [26••]. of 80 patients with wm, 63 (79%) patients were previously exposed to a covalent btki, with 41 being refractory to it [ 27 ••]. Pirtobrutinib (loxo 305) is the leading member of a new generation of btki that noncovalently binds btk, distant from the c481 residue. 17 emerging data from clinical trials of pirtobrutinib, 17 nemtabrutinib (mk1026, arq531), 24 and vecabrutinib (sns 062) 25 have shown clear clinical activity in patients whose disease carries the c481s mutant but also in patients without c481s mutation.

Characteristics Of Patients On Pirtobrutinib Trial Or Used For In Vitro Pirtobrutinib has been examined in the bruin study, a phase i ii, multicenter trial (nct03740529) assessing patients with r r b cell malignancies [26••]. of 80 patients with wm, 63 (79%) patients were previously exposed to a covalent btki, with 41 being refractory to it [ 27 ••]. Pirtobrutinib (loxo 305) is the leading member of a new generation of btki that noncovalently binds btk, distant from the c481 residue. 17 emerging data from clinical trials of pirtobrutinib, 17 nemtabrutinib (mk1026, arq531), 24 and vecabrutinib (sns 062) 25 have shown clear clinical activity in patients whose disease carries the c481s mutant but also in patients without c481s mutation.

Characteristics Of Patients On Pirtobrutinib Trial Or Used For In Vitro

Comments are closed.