6 Concept Of Work And Heat In Thermodynamics Youtube

06 Concept Of Heat And Work Thermodynamics Bsc 2nd Year Physical This is a basic introduction to the concepts of heat, work and temperature. you will come across those terms all the time in thermodynamics and it is quite. #chemsolute #bsc2ndyearclasses #heat #work #workandheattransferinthermodynamicslecture 01 👉 youtu.be rhnwjtuun3slecture 02 👉 youtu.be l87w0.

6 Work And Heat In Thermodynamics Sign Convention Of Work And Heat In chemistry we talked about the first law of thermodynamics as being the law of conservation of energy, and that's one way of looking at it, but physicists. Figure 12.6 the first law of thermodynamics is the conservation of energy principle stated for a system, where heat and work are the methods of transferring energy to and from a system. q represents the net heat transfer—it is the sum of all transfers of energy by heat into and out of the system. This page titled 3.3: work, heat, and internal energy is shared under a cc by 4.0 license and was authored, remixed, and or curated by openstax via source content that was edited to the style and standards of the libretexts platform. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. The gas is kept at a constant temperature t by keeping it in thermal equilibrium with a heat reservoir at that temperature. from equation 3.4 and the ideal gas law, w = ∫v 2 v 1 pdv = ∫v 2 v 1 (nrt v)dv. w = ∫ v 1 v 2 p d v = ∫ v 1 v 2 (n r t v) d v.
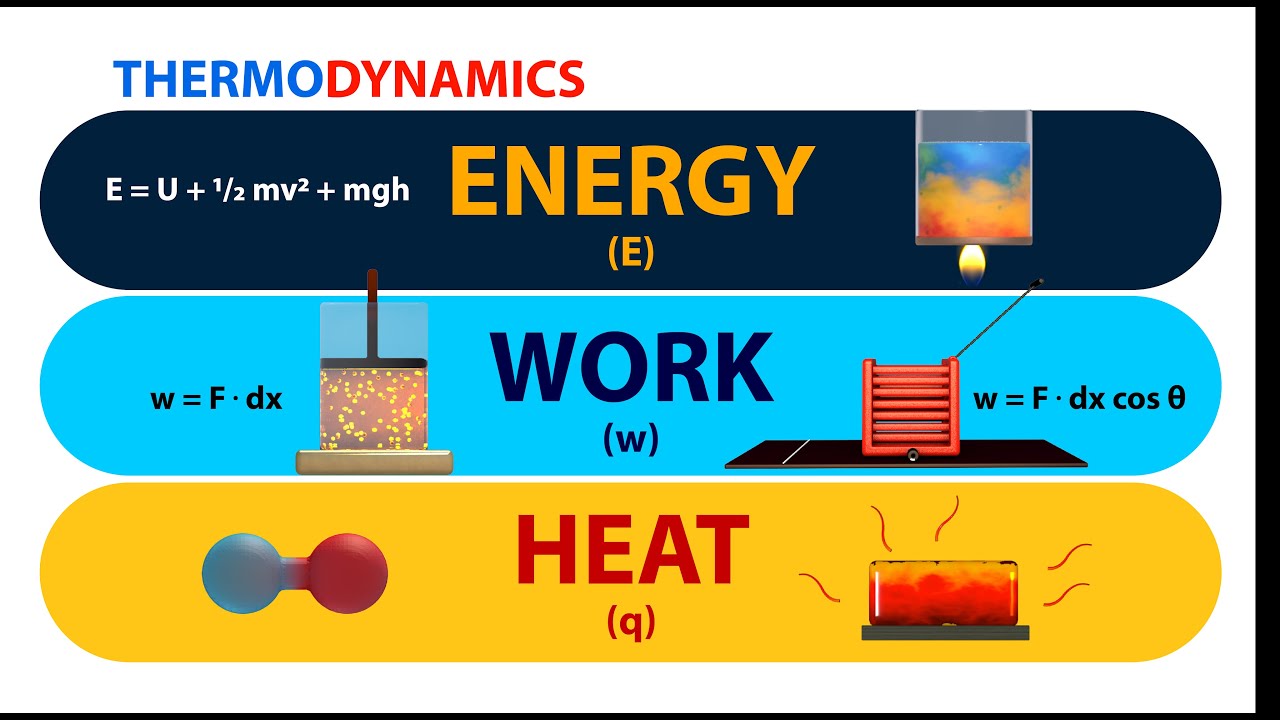
Thermodynamics Energy Work And Heat Animation Youtube This page titled 3.3: work, heat, and internal energy is shared under a cc by 4.0 license and was authored, remixed, and or curated by openstax via source content that was edited to the style and standards of the libretexts platform. positive (negative) work is done by a thermodynamic system when it expands (contracts) under an external pressure. The gas is kept at a constant temperature t by keeping it in thermal equilibrium with a heat reservoir at that temperature. from equation 3.4 and the ideal gas law, w = ∫v 2 v 1 pdv = ∫v 2 v 1 (nrt v)dv. w = ∫ v 1 v 2 p d v = ∫ v 1 v 2 (n r t v) d v. Heat is a form of energy, which flows due to difference in temperature. it flows from high temperature to low temperature. heat is a path function, ie, it depends on the path followed by the system to reach to the current state. sign convention: heat absorbed by the system is taken as positive and heat released by the system is taken as negative. This chemistry video tutorial provides a basic introduction into the first law of thermodynamics. it shows the relationship between internal energy, heat, a.

Lec114 物理 一 Work And Heat In Thermodynamics Youtube Heat is a form of energy, which flows due to difference in temperature. it flows from high temperature to low temperature. heat is a path function, ie, it depends on the path followed by the system to reach to the current state. sign convention: heat absorbed by the system is taken as positive and heat released by the system is taken as negative. This chemistry video tutorial provides a basic introduction into the first law of thermodynamics. it shows the relationship between internal energy, heat, a.

Comments are closed.