513g Request For Information
513g Request For Information Medical Device Academy The purpose of this guidance is to establish procedures for submitting, reviewing and responding to requests for information regarding the class in which a device has been classified or the. If, based solely on the information provided with a 513(g) request for information, the product at issue does not appear to be a "device" within the meaning of section 201(h) of the.

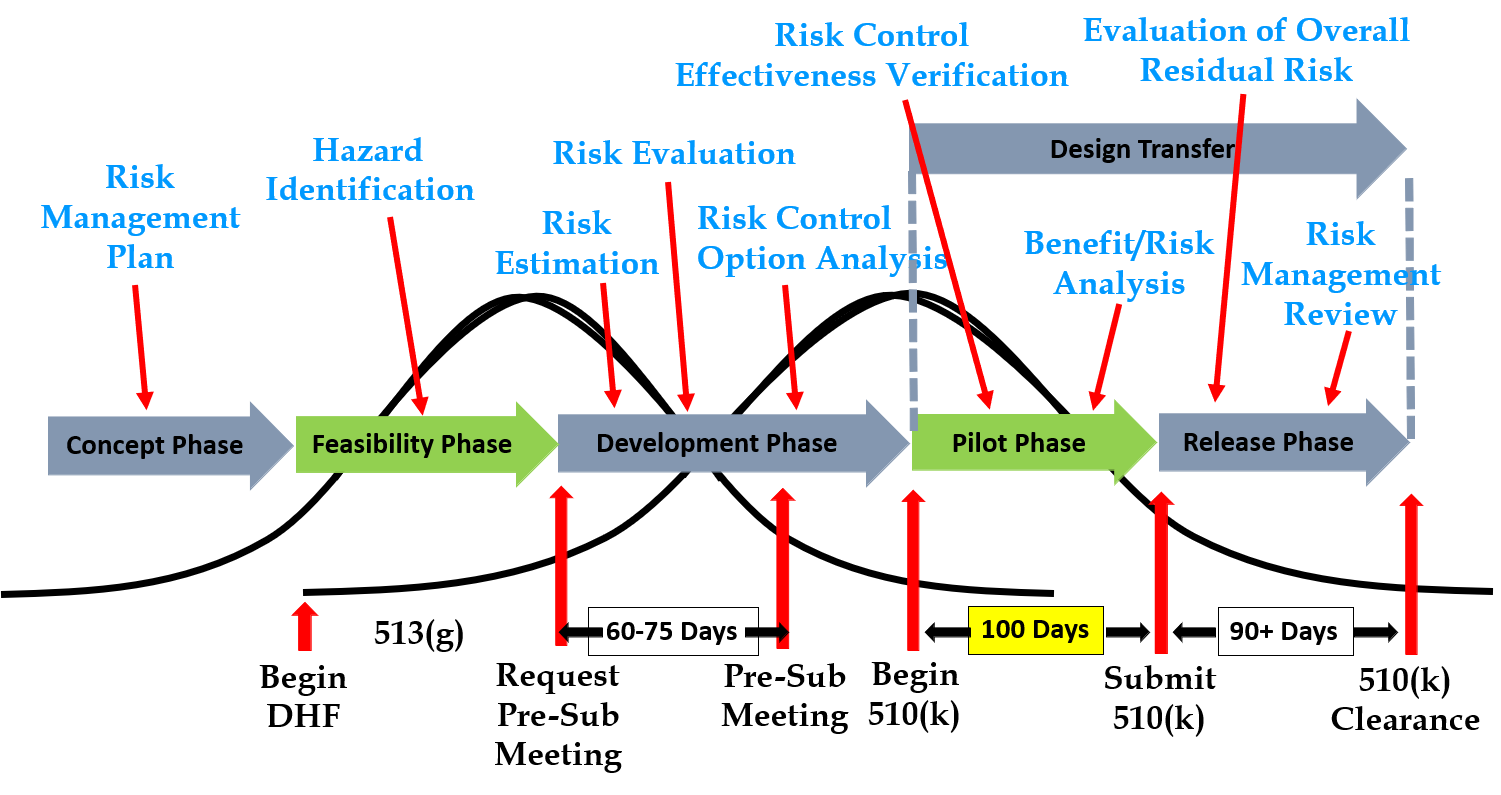
513g Request For Information Information to include in a 513(g) request. 1. cover letter 2. device description 3. intended use 4. labeling claims. 19. guidance: fda and industry procedures for section 513(g) requests for. A “513g” is a request for classification information from the fda. the reference is to a food, drug & cosmetic act section. the purpose of the submission is to ask the fda what product classification would be most appropriate for your device and what the appropriate regulatory pathway will be. the regulation requires the fda to provide a. Final. issued by: food and drug administration (fda) issue date: december 16, 2019 disclaimer: the contents of this database lack the force and effect of law, except as authorized by law (including medicare advantage rate announcements and advance notices) or as specifically incorporated into a contract. Fda 513(g) refers to a section of the food, drug, and cosmetic act that allows device manufacturers to request information from the fda regarding the regulatory status of a device. the primary objective is to gain clarification on the applicable regulatory requirements and determine the most appropriate pathway for bringing a medical device to.

Ppt 513 G S Requests For Information Powerpoint Presentation Free Final. issued by: food and drug administration (fda) issue date: december 16, 2019 disclaimer: the contents of this database lack the force and effect of law, except as authorized by law (including medicare advantage rate announcements and advance notices) or as specifically incorporated into a contract. Fda 513(g) refers to a section of the food, drug, and cosmetic act that allows device manufacturers to request information from the fda regarding the regulatory status of a device. the primary objective is to gain clarification on the applicable regulatory requirements and determine the most appropriate pathway for bringing a medical device to. Section 513 (g) of the federal food, drug, and cosmetic act provides a means for device manufacturers to obtain information about the food and drug administration’s views regarding the classification of a device. according to the legislation, a company can submit a written request to the secretary. the secretary will provide a written reply. A pre submission provides the submitter an opportunity to obtain fda feedback prior to a planned medical device premarket submission. a 513 (g) request for information is a means of obtaining fda’s views about the classification and regulatory requirements for a particular device. estar templates. as we have previously explained in our blog.

Comments are closed.