4 3 Limiting Reactant Theoretical Yield And Percent

4 3 Limiting Reactant Theoretical Yield Percent Yield Youtube The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100 to give a percentage: percent yield = actual yield (g) theoretical yield(g) × 100%. the method used to calculate the percent yield of a reaction is illustrated in example 4.3.4. The actual yield is the amount of product that we finally obtain. thus, the percent yield is the ratio of the actual yield to the theoretical yield mutiplied by 100. what is the formula for percent yield? percent yield = (actual yield theoretical yield) x 100. if the theoretical yield is 14.8g for a reaction and the amount of product obtained.

4 3 Limiting Reactant Theoretical Yield And Percent This article explains the concept of limiting reagents and percent yield in chemical reactions. Limiting reactant. percent yield. theoretical yield. 6.2: limiting reactant, theoretical yield, and percent yield is shared under a not declared license and was authored, remixed, and or curated by libretexts. when reactions are carried out using less than stoichiometric quantities of reactants, the amount of product generated will be. Ty the reactant that gives the smallest amount of product.th. l. iting reagent determines the am. unt of product formed.4. identify the theoretical yield:the theoretical yield is the amo. nt. f the product (in g) forme. from the limiting reagent.5. identify the actual yield:the actual yield is the amount of t. Calculate the percent yield, determine the limiting reactant, write a balanced equation, calculate the theoretical yield. a) write a balanced equation, determine the limiting reactant, calculate the theoretical yield, calculate the percent yield. a welder has 1.873 × 10^2 g fe2o3 and 94.51 g al in his welding kit.
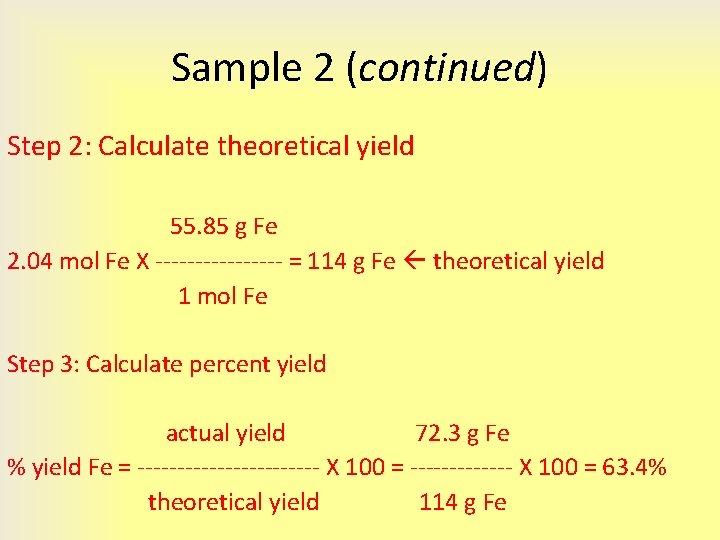
4 3 Limiting Reactant Theoretical Yield And Percent Ty the reactant that gives the smallest amount of product.th. l. iting reagent determines the am. unt of product formed.4. identify the theoretical yield:the theoretical yield is the amo. nt. f the product (in g) forme. from the limiting reagent.5. identify the actual yield:the actual yield is the amount of t. Calculate the percent yield, determine the limiting reactant, write a balanced equation, calculate the theoretical yield. a) write a balanced equation, determine the limiting reactant, calculate the theoretical yield, calculate the percent yield. a welder has 1.873 × 10^2 g fe2o3 and 94.51 g al in his welding kit. The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, expressed as a percentage. 4.3: limiting reactant, theoretical yield, and percent yield is shared under a not declared license and was authored, remixed, and or curated by libretexts. the stoichiometry of a balanced chemical equation identifies the. Figure 1. sandwich making can illustrate the concepts of limiting and excess reactants. consider this concept now with regard to a chemical process, the reaction of hydrogen with chlorine to yield hydrogen chloride: h2(s) cl2(g) 2hcl(g) the balanced equation shows the hydrogen and chlorine react in a 1:1 ratio.

4 3 Limiting Reactant Theoretical Yield Percent Yield Youtube The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, expressed as a percentage. 4.3: limiting reactant, theoretical yield, and percent yield is shared under a not declared license and was authored, remixed, and or curated by libretexts. the stoichiometry of a balanced chemical equation identifies the. Figure 1. sandwich making can illustrate the concepts of limiting and excess reactants. consider this concept now with regard to a chemical process, the reaction of hydrogen with chlorine to yield hydrogen chloride: h2(s) cl2(g) 2hcl(g) the balanced equation shows the hydrogen and chlorine react in a 1:1 ratio.

4 3 Limiting Reactant Theoretical Yield And Percent

Comments are closed.